Nutr Res Pract.
2015 Dec;9(6):592-598. 10.4162/nrp.2015.9.6.592.
The micosporine-like amino acids-rich aqueous methanol extract of laver (Porphyra yezoensis) inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Affiliations
-
- 1Department of Chemistry, Incheon National University, 119 Academy-ro, Yeonsu-gu, Incheon 406-772, Korea. eunmi@inu.ac.kr
- 2Department of Marine Sciences, Incheon National University, Incheon 406-772, Korea.
- KMID: 2313882
- DOI: http://doi.org/10.4162/nrp.2015.9.6.592
Abstract
- BACKGROUND/OBJECTIVES
Increased mass of adipose tissue in obese persons is caused by excessive adipogenesis, which is elaborately controlled by an array of transcription factors. Inhibition of adipogenesis by diverse plant-derived substances has been explored. The aim of the current study was to examine the effects of the aqueous methanol extract of laver (Porphyra yezoensis) on adipogenesis and apoptosis in 3T3-L1 adipocytes and to investigate the mechanism underlying the effect of the laver extract.
MATERIALS/METHODS
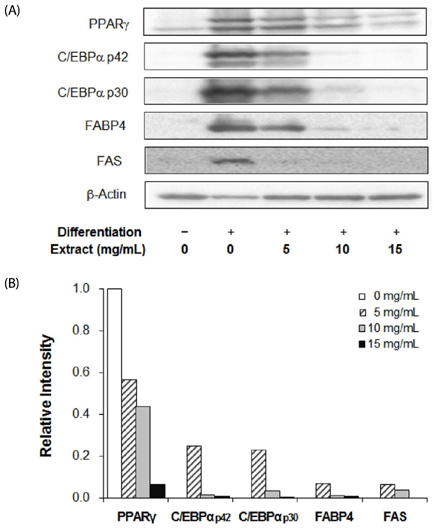
3T3-L1 cells were treated with various concentrations of laver extract in differentiation medium. Lipid accumulation, expression of adipogenic proteins, including CCAAT enhancer-binding protein alpha, peroxisome proliferator-activated receptor gamma, fatty acid binding protein 4, and fatty acid synthase, cell viability, apoptosis, and the total content and the ratio of reduced to oxidized forms of glutathione (GSH/GSSG) were analyzed.
RESULTS
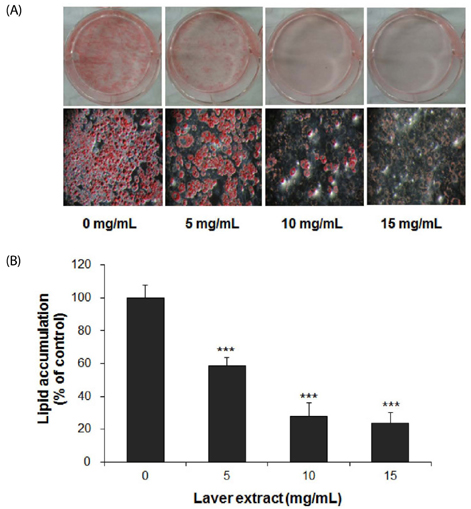
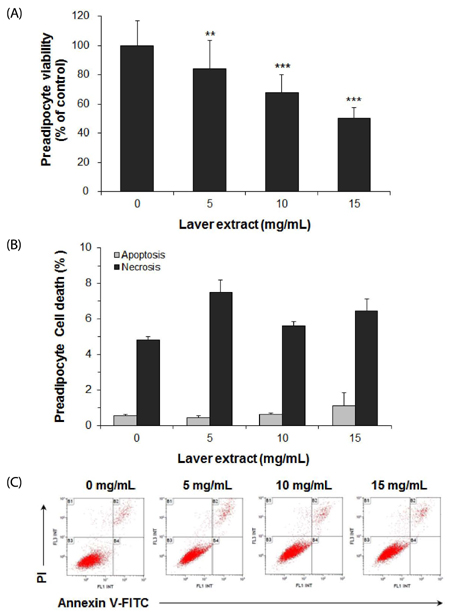
Treatment with laver extract resulted in a significant decrease in lipid accumulation in 3T3-L1 adipocytes, which showed correlation with a reduction in expression of adipogenic proteins. Treatment with laver extract also resulted in a decrease in the viability of preadipocytes and an increase in the apoptosis of mature adipocytes. Treatment with laver extract led to exacerbated depletion of cellular glutathione and abolished the transient increase in GSH/GSSG ratio during adipogenesis in 3T3-L1 adipocytes.
CONCLUSION
Results of our study demonstrated that treatment with the laver extract caused inhibition of adipogenesis, a decrease in proliferation of preadipocytes, and an increase in the apoptosis of mature adipocytes. It appears that these effects were caused by increasing oxidative stress, as demonstrated by the depletion and oxidation of the cellular glutathione pool in the extract-treated adipocytes. Our results suggest that a prooxidant role of laver extract is associated with its antiadipogenic and proapoptotic effects.
Keyword
MeSH Terms
Figure
Reference
-
1. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001; 414:799–806.
Article2. Camp HS, Ren D, Leff T. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol Med. 2002; 8:442–447.
Article3. Couillard C, Mauriège P, Imbeault P, Prud'homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int J Obes Relat Metab Disord. 2000; 24:782–788.
Article4. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006; 4:263–273.
Article5. Lowe CE, O'Rahilly S, Rochford JJ. Adipogenesis at a glance. J Cell Sci. 2011; 124:2681–2686.
Article6. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998; 78:783–809.
Article7. Rayalam S, Della-Fera MA, Baile CA. Phytochemicals and regulation of the adipocyte life cycle. J Nutr Biochem. 2008; 19:717–726.
Article8. Lee JC, Hou MF, Huang HW, Chang FR, Yeh CC, Tang JY, Chang HW. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell Int. 2013; 13:55.
Article9. Faulkner DJ. Marine natural products. Nat Prod Rep. 2002; 19:1–48.
Article10. Yuan YV, Walsh NA. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem Toxicol. 2006; 44:1144–1150.
Article11. Gröniger A, Sinha RP, Klisch M, Häder DP. Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae--a database. J Photochem Photobiol B. 2000; 58:115–122.
Article12. Sinha RP, Singh SP, Häder DP. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J Photochem Photobiol B. 2007; 89:29–35.
Article13. Esteban R, Martínez B, Fernández-Marín B, María Becerril J, García-Plazaola JI. Carotenoid composition in Rhodophyta: insights into xanthophyll regulation in Corallina elongata. Eur J Phycol. 2009; 44:221–230.
Article14. Olsen EK, Hansen E, Isaksson J, Andersen JH. Cellular antioxidant effect of four bromophenols from the red algae, Vertebrata lanosa. Mar Drugs. 2013; 11:2769–2784.
Article15. Wang H, Fu XM, Han CC. The potential applications of marine bioactives against diabetes and obesity. Am J Mar Sci. 2014; 2:1–8.16. Kim KJ, Lee OH, Lee BY. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010; 86:791–797.
Article17. Maeda H, Hosokawa M, Sashima T, Takahashi N, Kawada T, Miyashita K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int J Mol Med. 2006; 18:147–152.
Article18. Jeon HJ, Seo MJ, Choi HS, Lee OH, Lee BY. Gelidium elegans, an edible red seaweed, and hesperidin inhibit lipid accumulation and production of reactive oxygen species and reactive nitrogen species in 3T3-L1 and RAW264.7 cells. Phytother Res. 2014; 28:1701–1709.
Article19. Kim HM, Kang SI, Shin HS, Ko HC, Hong YS, Kang SW, Yoon SA, Kim JH, Kim SJ. Anti-obesity effect of Komulkosiraegi [Gracilaria vermiculophylla (Ohmi) Papenfuss] extract in 3T3-L1 cells. Food Sci Biotechnol. 2012; 21:83–89.
Article20. Gironi F, Piemonte V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem Eng Res Des. 2011; 89:857–862.
Article21. Volkmann M, Gorbushina AA. A broadly applicable method for extraction and characterization of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiol Lett. 2006; 255:286–295.
Article22. Klisch M, Häder DP. Wavelength dependence of mycosporine-like amino acid synthesis in Gyrodinium dorsum. J Photochem Photobiol B. 2002; 66:60–66.
Article23. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am K Enol Vitic. 1965; 16:144–158.24. Ramírez-Zacarías JL, Castro-Muñozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992; 97:493–497.
Article25. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article26. Neuschwander-Tetri BA, Roll FJ. Glutathione measurement by high-performance liquid chromatography separation and fluorometric detection of the glutathione-orthophthalaldehyde adduct. Anal Biochem. 1989; 179:236–241.
Article27. Kim S, You DH, Han T, Choi EM. Modulation of viability and apoptosis of UVB-exposed human keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). J Photochem Photobiol B. 2014; 141:301–307.
Article28. Zubia M, Fabre MS, Kerjean V, Deslandes E. Antioxidant and cytotoxic activities of some red algae (Rhodophyta) from Brittany coasts (France). Bot Mar. 2009; 52:268–277.
Article29. Hwang ES, Thi ND. Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (Porphyra tenera). Prev Nutr Food Sci. 2014; 19:40–48.
Article30. Landete JM. Updated knowledge about polyphenols: functions, bioavailability, metabolism, and health. Crit Rev Food Sci Nutr. 2012; 52:936–948.
Article31. Martin-Fernandez C, Bales J, Hodgkinson C, Welman A, Welham MJ, Dive C, Morrow CJ. Blocking phosphoinositide 3-kinase activity in colorectal cancer cells reduces proliferation but does not increase apoptosis alone or in combination with cytotoxic drugs. Mol Cancer Res. 2009; 7:955–965.
Article32. Hamtiaux L, Hansoulle L, Dauguet N, Muccioli GG, Gallez B, Lambert DM. Increasing antiproliferative properties of endocannabinoids in N1E-115 neuroblastoma cells through inhibition of their metabolism. PLoS One. 2011; 6:e26823.
Article33. Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008; 22:1367–1371.
Article34. Yang JY, Della-Fera MA, Rayalam S, Ambati S, Hartzell DL, Park HJ, Baile CA. Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008; 82:1032–1039.
Article35. Kim H, Hiraishi A, Tsuchiya K, Sakamoto K. (-) Epigallocatechin gallate suppresses the differentiation of 3T3-L1 preadipocytes through transcription factors FoxO1 and SREBP1c. Cytotechnology. 2010; 62:245–255.
Article36. Andersen C, Rayalam S, Della-Fera MA, Baile CA. Phytochemicals and adipogenesis. Biofactors. 2010; 36:415–422.
Article37. Liu GS, Chan EC, Higuchi M, Dusting GJ, Jiang F. Redox mechanisms in regulation of adipocyte differentiation: beyond a general stress response. Cells. 2012; 1:976–993.
Article38. Gummersbach C, Hemmrich K, Kröncke KD, Suschek CV, Fehsel K, Pallua N. New aspects of adipogenesis: radicals and oxidative stress. Differentiation. 2009; 77:115–120.
Article39. Mytilineou C, Kramer BC, Yabut JA. Glutathione depletion and oxidative stress. Parkinsonism Relat Disord. 2002; 8:385–387.
Article40. Torres-Ramrez N, Baiza-Gutman LA, García-Macedo R, Ortega-Camarillo C, Contreras-Ramos A, Medina-Navarro R, Cruz M, Ibáñez-Hernández MÁ, Díaz-Flores M. Nicotinamide, a glucose-6-phosphate dehydrogenase non-competitive mixed inhibitor, modifies redox balance and lipid accumulation in 3T3-L1 cells. Life Sci. 2013; 93:975–985.
Article41. Ducluzeau PH, Priou M, Weitheimer M, Flamment M, Duluc L, Iacobazi F, Soleti R, Simard G, Durand A, Rieusset J, Andriantsitohaina R, Malthièry Y. Dynamic regulation of mitochondrial network and oxidative functions during 3T3-L1 fat cell differentiation. J Physiol Biochem. 2011; 67:285–296.
Article42. Maher J, Yamamoto M. The rise of antioxidant signaling--the evolution and hormetic actions of Nrf2. Toxicol Appl Pharmacol. 2010; 244:4–15.
Article43. Hou Y, Xue P, Bai Y, Liu D, Woods CG, Yarborough K, Fu J, Zhang Q, Sun G, Collins S, Chan JY, Yamamoto M, Andersen ME, Pi J. Nuclear factor erythroid-derived factor 2-related factor 2 regulates transcription of CCAAT/enhancer-binding protein beta during adipogenesis. Free Radic Biol Med. 2012; 52:462–472.
Article44. Findeisen HM, Pearson KJ, Gizard F, Zhao Y, Qing H, Jones KL, Cohn D, Heywood EB, de Cabo R, Bruemmer D. Oxidative stress accumulates in adipose tissue during aging and inhibits adipogenesis. PLoS One. 2011; 6:e18532.
Article45. Tao L, Park JY, Lambert JD. Differential prooxidative effects of the green tea polyphenol, (-)-epigallocatechin-3-gallate, in normal and oral cancer cells are related to differences in sirtuin 3 signaling. Mol Nutr Food Res. 2015; 59:203–211.
Article46. Kucinska M, Piotrowska H, Luczak MW, Mikula-Pietrasik J, Ksiazek K, Wozniak M, Wierzchowski M, Dudka J, Jäger W, Murias M. Effects of hydroxylated resveratrol analogs on oxidative stress and cancer cells death in human acute T cell leukemia cell line: prooxidative potential of hydroxylated resveratrol analogs. Chem Biol Interact. 2014; 209:96–110.
Article47. Wang CT, Chang HH, Hsiao CH, Lee MJ, Ku HC, Hu YJ, Kao YH. The effects of green tea (-)-epigallocatechin-3-gallate on reactive oxygen species in 3T3-L1 preadipocytes and adipocytes depend on the glutathione and 67 kDa laminin receptor pathways. Mol Nutr Food Res. 2009; 53:349–360.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Soluble extract of soybean fermented with Aspergillus oryzae GB107 inhibits fat accumulation in cultured 3T3-L1 adipocytes
- Psidium guajava L. leaf extract inhibits adipocyte differentiation and improves insulin sensitivity in 3T3-L1 cells
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3