Nutr Res Pract.
2015 Oct;9(5):451-458. 10.4162/nrp.2015.9.5.451.
Hematopoietic effect of deer antler extract fermented by Bacillus subtilis on murine marrow cells
- Affiliations
-
- 1Department of Food and Nutrition, Korea University, 5 Anam-dong, Sungbuk-gu, Seoul 136-713, Korea. suh1960@korea.ac.kr
- 2Department of Food Science and Technology, Seoul Women's University, Seoul 139-774, Korea.
- 3Food Quality & Safety Center, Agency for Korea Food Cluster, Gwacheon 427-719, Korea.
- KMID: 2313864
- DOI: http://doi.org/10.4162/nrp.2015.9.5.451
Abstract
- BACKGROUND/OBJECTIVES
We examined the chemical composition and the effect of fermented deer antler on hematopoietic factors in bone marrow cells.
MATERIALS/METHODS
For the preparation of fermented deer antler extract (FAB), fermentation was carried out using Bacillus subtilis at 30degrees C for 7 days. The hematopoietic effect of FAB was investigated hematopoietic factors in marrow cells.
RESULTS
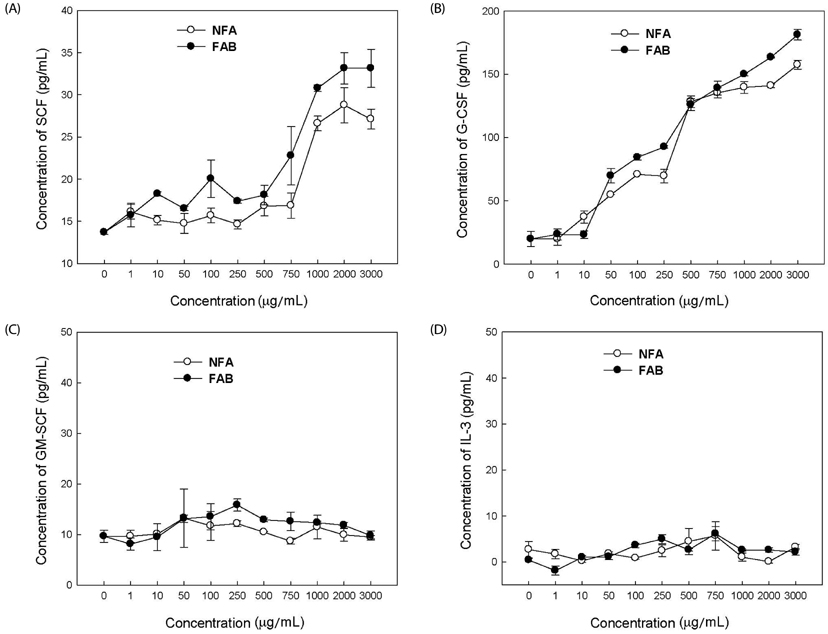
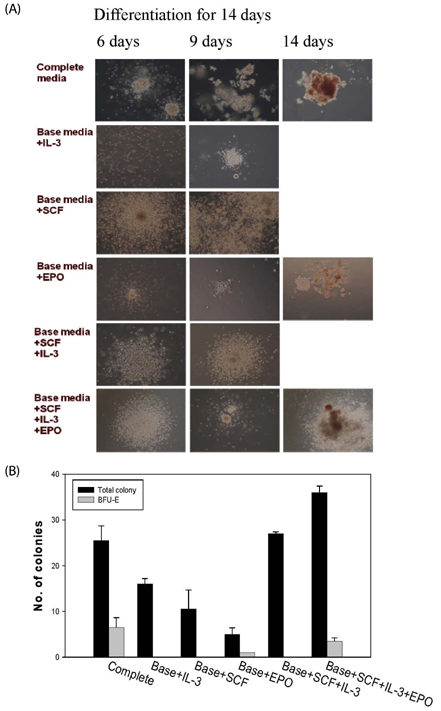
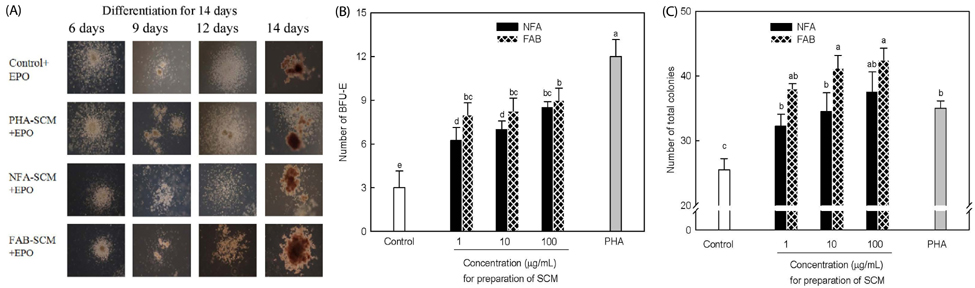
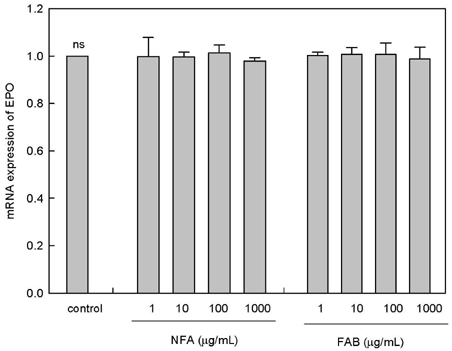
The contents of total sugar, sulfated glycosaminoglycans, and uronic acid and the dry weight gradually increased with fermentation time. The sialic acid content (from 0.14 mg/mL to 0.54 mg/mL) was the highest on the 4th day of fermentation after which it decreased. The proliferating activity of bone marrow cells increased with fermentation times. The levels of various hematopoietic growth factors were determined to verify the beneficial effect of deer antler extract fermented by B. subtilis on hematopoiesis. FAB increased the number of stem cell factors and granulocyte colony-stimulating factor in bone marrow cells. In addition, FAB augmented the burst-forming unit erythroid and total colonies in splenocyte-conditioned medium compared with non-fermented antler extract (NFA). However, FAB did not affect the mRNA levels of erythropoietin, an important factor for erythropoiesis.
CONCLUSIONS
FAB, like NFA, did not directly affect hematopoiesis, but contributed to hematopoiesis by stimulating the production of hematopoietic factors.
Keyword
MeSH Terms
-
Animals
Antlers*
Bacillus subtilis*
Bacillus*
Bone Marrow Cells
Bone Marrow*
Deer*
Erythropoiesis
Erythropoietin
Fermentation
Glycosaminoglycans
Granulocyte Colony-Stimulating Factor
Hematopoiesis
Intercellular Signaling Peptides and Proteins
N-Acetylneuraminic Acid
RNA, Messenger
Stem Cell Factor
Erythropoietin
Glycosaminoglycans
Granulocyte Colony-Stimulating Factor
Intercellular Signaling Peptides and Proteins
N-Acetylneuraminic Acid
RNA, Messenger
Stem Cell Factor
Figure
Reference
-
1. Henning GT, Schild SE, Stafford SL, Donohue JH, Burch PA, Haddock MG, Gunderson LL. Results of irradiation or chemoirradiation for primary unresectable, locally recurrent, or grossly incomplete resection of gastric adenocarcinoma. Int J Radiat Oncol Biol Phys. 2000; 46:109–118.
Article2. Mitchell SE, Mendenhall WM, Zlotecki RA, Carroll RR. Squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2001; 49:1007–1013.
Article3. Ha YW, Jeon BT, Moon SH, Kim YS. Comparison of biochemical components among different fodders-treated antlers. Korean J Pharmacogn. 2003; 34:40–44.4. Hassan HT, Zander A. Stem cell factor as a survival and growth factor in human normal and malignant hematopoiesis. Acta Haematol. 1996; 95:257–262.
Article5. Ivankina NF, Isay SV, Busarova NG, Mischenko T. Prostaglandin-like activity, fatty acid and phospholipid composition of sika deer (Cervus nippon) antlers at different growth stages. Comp Biochem Physiol B. 1993; 106:159–162.
Article6. Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, Jhon GJ, Han SY. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler). Chem Pharm Bull (Tokyo). 2004; 52:874–878.
Article7. Yang HO, Park JS, Cho SH, Yoon JY, Kim MG, Jhon GJ, Han SY, Kim SH. Stimulatory effects of monoacetyldiglycerides on hematopoiesis. Biol Pharm Bull. 2004; 27:1121–1125.
Article8. Wang BX, Zhao XH, Qi SB, Kaneko S, Hattori M, Namba T, Nomura Y. Effects of repeated administration of deer antler extract on biochemical changes related to aging in senescence-accelerated mice. Chem Pharm Bull (Tokyo). 1988; 36:2587–2592.
Article9. Zhao QC, Kiyohara H, Nagai T, Yamada H. Structure of the complement-activating proteoglycan from the pilose antler of Cervus nippon Temminck. Carbohydr Res. 1992; 230:361–372.
Article10. Song SK. Influence of deer horn on erythropoietin activity and radioactive iron uptake in rabbits. J Cathol Med Coll. 1970; 18:51–60.11. Li JJ, Li Z, Gu LJ, Wang YB, Lee MR, Sung CK. Aqueous extract of red deer antler promotes hair growth by regulating the hair cycle and cell proliferation in hair follicles. ScientificWorldJournal. 2014; 2014:878162.
Article12. Kim KW, Park SW. A study on the hemopoietic action of deer horn extract. Korean Biochem J. 1982; 15:151–157.13. Lee MR, Kim HH, Jo HH, Kang HJ, Gu L, Ly SY, Lee CH, Kim SM, Yang SA, Mo EK, Sung CK. Hemopoietic effect of extracts from four parts of deer antler on phenylhydrazine-induced hemolytic anemia in female rats. J Korean Soc Food Sci Nutr. 2009; 38:1718–1723.
Article14. Lee SH, Suh HJ, Lee HS, Park Y, Park JW, Jung EY. Hematopoietic effect of Bacillus subtilis-fermented antler extract on phenylhydrazine-induced hemolytic anemia in Sprague-Dawley rats. J Med Food. 2012; 15:774–780.
Article15. Kim MK, Jung EY, Lee HS, Shin KS, Kim YK, Ra KS, Park CS, Woo MJ, Lee SH, Kim JS, Suh HJ. Isolation of strain for the preparation of the fermented antler and its physiological activities. J Korean Soc Food Sci Nutr. 2009; 38:1237–1242.
Article16. Lee HS, Kim MK, Kim YK, Jung EY, Park CS, Woo MJ, Lee SH, Kim JS, Suh HJ. Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by antler and fermented antler using Cordyceps militaris. J Ethnopharmacol. 2011; 133:710–717.
Article17. DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956; 28:350–356.
Article18. Chen H, Zhang M, Xie B. Quantification of uronic acids in tea polysaccharide conjugates and their antioxidant properties. J Agric Food Chem. 2004; 52:3333–3336.
Article19. Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982; 9:247–248.
Article20. Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959; 234:1971–1975.
Article21. Yu KW, Kiyohara H, Matsumoto T, Yang HC, Yamada H. Intestinal immune system modulating polysaccharides from rhizomes of Atractylodes lancea. Planta Med. 1998; 64:714–719.
Article22. Liao HF, Chen YJ, Yang YC. A novel polysaccharide of black soybean promotes myelopoiesis and reconstitutes bone marrow after 5-flurouracil- and irradiation-induced myelosuppression. Life Sci. 2005; 77:400–413.
Article23. Kouro T, Yokota T, Welner R, Kincade PW. Measurement of natural killer cell progenitor activity in culture. Curr Protoc Immunol. 2005; Chapter 22:Unit 22F.3.
Article24. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.
Article25. Wang SY, Chen LY, Tsai TF, Su TS, Choo KB, Ho CK. Constitutive production of colony-stimulating factors by human hepatoma cell lines: possible correlation with cell differentiation. Exp Hematol. 1996; 24:437–444.26. Mecucci C, Cassiman JJ, van der Schueren B, Dewolf-Peeters C, Falini B, van den Berghe H. Phytohemagglutinin-conditioned medium modulates adherence properties and morphology of hairy cells. Leuk Res. 1986; 10:1091–1099.
Article27. Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996; 87:30–37.
Article28. Brinda R, Parvathy S. Ethnobotanical medicines of anaimalai union pollachi taluk, Coimbatore district, tamilnadu. Anc Sci Life. 2003; 22:166–168.29. Wu F, Li H, Jin L, Li X, Ma Y, You J, Li S, Xu Y. Deer antler base as a traditional Chinese medicine: a review of its traditional uses, chemistry and pharmacology. J Ethnopharmacol. 2013; 145:403–415.
Article30. Ketley NJ, Newland AC. Haemopoietic growth factors. Postgrad Med J. 1997; 73:215–221.
Article31. Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001; 98:4044–4049.
Article32. Haroon ZA, Amin K, Jiang X, Arcasoy MO. A novel role for erythropoietin during fibrin-induced wound-healing response. Am J Pathol. 2003; 163:993–1000.
Article33. Su F, Li H, Wang Y, Huang Y, Xiao S, Xiao Y. Protein component extraction and its bioactivity determination of Sika deer antler base. Anim Sci Vet Med. 2001; 18:18–20.34. Kong Q, Zhang Y, Wang XJ, Ma M, Chen L, Cui J. A study of IIRWP's increasing effects on WBC number. Chin J Mod Appl Pharm. 1998; 15:12–15.35. Tonelli Q, Meints RH. Sialic acid: a specific role in hematopoietic spleen colony formation. J Supramol Struct. 1978; 8:67–78.
Article36. Park SY. Hemato poietic effect of deer antler extract fermented by Bacillus subtilis on primary cell culture of bone marrow and iron deficiency anemic rat [Master's thesis]. Seoul: Korea University;2012.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Deer(Cervus nippon) Antler Extracts on Differentiation of MC3T3 Cells
- Anti-hyperlipidemic effect of soybean extract fermented by Bacillus subtilis MORI in db/db mice
- Fermented antler extract enhances the viability and interleukin-12 production of spleen cells
- Effect of Deer Antler Drink Supplementation on Blood Pressure, Blood Glucose and Lymphocyte DNA Damage in Type 2 Diabetic Patients
- Effects of Deer Antler Extract on Serum IGF-I, Bone Growth and Splenocyte Proliferation in Growing Rats