Nutr Res Pract.
2015 Apr;9(2):123-128. 10.4162/nrp.2015.9.2.123.
Protective role of oligonol from oxidative stress-induced inflammation in C6 glial cell
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, Busandaehak-ro 63 beon-gil, Geumjeong-gu, Busan 609-735, Korea. ejcho@pusan.ac.kr
- 2Amino Up Chemical Co., Ltd, Sapporo 004-0839, Japan.
- 3Institute of Natural Medicine, University of Toyama, Toyama 930-0194, Japan.
- KMID: 2313819
- DOI: http://doi.org/10.4162/nrp.2015.9.2.123
Abstract
- BACKGROUND/OBJECTIVES
Natural products or active components with a protective effect against oxidative stress have attracted significant attention for prevention and treatment of degenerative disease. Oligonol is a low molecular weight polyphenol containing catechin-type monomers and oligomers derived from Litchi chinensis Sonn. We investigated the protective effect and its related mechanism of oligonol against oxidative stress.
MATERIALS/METHODS
Oxidative stress in C6 glial cells was induced by hydrogen peroxide (H2O2) and the protective effects of oligonol on cell viability, nitric oxide (NO) and reactive oxygen species (ROS) synthesis, and mRNA expression related to oxidative stress were determined.
RESULTS
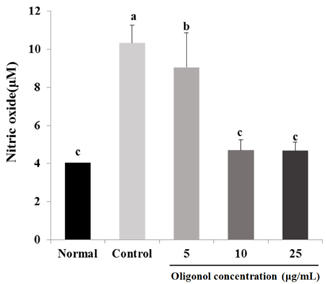
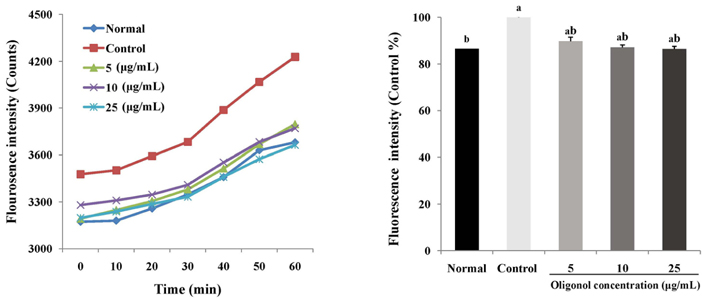
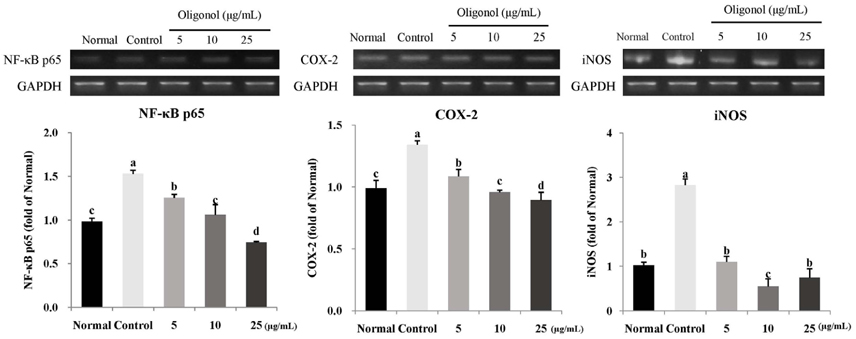
Treatment with oligonol inhibited NO and ROS formation under cellular oxidative stress in C6 glial cells. In addition, it recovered cell viability in a dose dependent-manner. Treatment with oligonol also resulted in down-regulated mRNA expression related to oxidative stress, nuclear factor kappa-B (NF-kappaB) p65, cyclooxygenase-2 (COX-2), and inducible nitric oxide synthase (iNOS), compared with the control group treated with H2O2. In particular, expression of NF-kappaB p65, COX-2, and iNOS was effectively reduced to the normal level by treatment with 10 microg/mL and 25 microg/mL of oligonol.
CONCLUSIONS
These results indicate that oligonol has protective activity against oxidative stress-induced inflammation. Oligonol might be a promising agent for treatment of degenerative diseases through inhibition of ROS formation and NF-kappaB pathway gene expression.
Keyword
MeSH Terms
-
Biological Products
Cell Survival
Cyclooxygenase 2
Gene Expression
Hydrogen Peroxide
Inflammation*
Litchi
Molecular Weight
Neuroglia*
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
Oxidative Stress
Reactive Oxygen Species
RNA, Messenger
Biological Products
Cyclooxygenase 2
Hydrogen Peroxide
NF-kappa B
Nitric Oxide
Nitric Oxide Synthase Type II
RNA, Messenger
Reactive Oxygen Species
Figure
Reference
-
1. Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993; 262:689–695.
Article2. Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005; 37:289–305.
Article3. Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003; 304:1–7.
Article4. Cirino G, Distrutti E, Wallace JL. Nitric oxide and inflammation. Inflamm Allergy Drug Targets. 2006; 5:115–119.
Article5. Pahan K, Raymond JR, Singh I. Inhibition of phosphatidylinositol 3-kinase induces nitric-oxide synthase in lipopolysaccharide- or cytokine-stimulated C6 glial cells. J Biol Chem. 1999; 274:7528–7536.
Article6. Jiang G, Lin S, Wen L, Jiang Y, Zhao M, Chen F, Prasad KN, Duan X, Yang B. Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chem. 2013; 136:563–568.
Article7. Wang L, Lou G, Ma Z, Liu X. Chemical constituents with antioxidant activities from litchi (Litchi chinensis Sonn.) seeds. Food Chem. 2011; 126:1081–1087.
Article8. Ong PK, Acree TE. Similarities in the aroma chemistry of Gewürztraminer variety wines and lychee (Litchi chinesis sonn.) fruit. J Agric Food Chem. 1999; 47:665–670.
Article9. Yang B, Wang J, Zhao M, Liu Y, Wang W, Jiang Y. Identification of polysaccharides from pericarp tissues of litchi (Litchi chinensis Sonn.) fruit in relation to their antioxidant activities. Carbohydr Res. 2006; 341:634–638.
Article10. Bhoopat L, Srichairatanakool S, Kanjanapothi D, Taesotikul T, Thananchai H, Bhoopat T. Hepatoprotective effects of lychee (Litchi chinensis Sonn.): a combination of antioxidant and anti-apoptotic activities. J Ethnopharmacol. 2011; 136:55–66.
Article11. Sarni-Manchado P, Le Roux E, Le Guernevé C, Lozano Y, Cheynier V. Phenolic composition of litchi fruit pericarp. J Agric Food Chem. 2000; 48:5995–6002.
Article12. Fujii H, Nishioka H, Wakame K, Magnuson BA, Roberts A. Acute, subchronic and genotoxicity studies conducted with Oligonol, an oligomerized polyphenol formulated from lychee and green tea extracts. Food Chem Toxicol. 2008; 46:3553–3562.
Article13. Noh JS, Park CH, Yokozawa T. Treatment with oligonol, a low-molecular polyphenol derived from lychee fruit, attenuates diabetes-induced hepatic damage through regulation of oxidative stress and lipid metabolism. Br J Nutr. 2011; 106:1013–1022.
Article14. Kundu JK, Chang EJ, Fujii H, Sun B, Surh YJ. Oligonol inhibits UVB-induced COX-2 expression in HR-1 hairless mouse skin--AP-1 and C/EBP as potential upstream targets. Photochem Photobiol. 2008; 84:399–406.
Article15. Zhang XH, Yokoo H, Nishioka H, Fujii H, Matsuda N, Hayashi T, Hattori Y. Beneficial effect of the oligomerized polyphenol oligonol on high glucose-induced changes in eNOS phosphorylation and dephosphorylation in endothelial cells. Br J Pharmacol. 2010; 159:928–938.
Article16. Ialenti A, Ianaro A, Moncada S, Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 1992; 211:177–182.
Article17. Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res. 2001; 480-481:243–268.
Article18. Dobashi K, Pahan K, Chahal A, Singh I. Modulation of endogenous antioxidant enzymes by nitric oxide in rat C6 glial cells. J Neurochem. 1997; 68:1896–1903.
Article19. Garcion E, Sindji L, Montero-Menei C, Andre C, Brachet P, Darcy F. Expression of inducible nitric oxide synthase during rat brain inflammation: regulation by 1,25-dihydroxyvitamin D3. Glia. 1998; 22:282–294.
Article20. Syapin PJ, Militante JD, Garrett DK, Ren L. Cytokine-induced iNOS expression in C6 glial cells: transcriptional inhibition by ethanol. J Pharmacol Exp Ther. 2001; 298:744–752.21. Peterson LJ, Flood PM. Oxidative stress and microglial cells in Parkinson's disease. Mediators Inflamm. 2012; 2012:401264.
Article22. McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997; 112:1022–1027.
Article23. Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004; 75:639–653.
Article24. Grandison L, Nolan GP, Pfaff DW. Activation of the transcription factor NF-κB in GH3 pituitary cells. Mol Cell Endocrinol. 1994; 106:9–15.25. O'Neill LA, Kaltschmidt C. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997; 20:252–258.26. Gookin JL, Chiang S, Allen J, Armstrong MU, Stauffer SH, Finnegan C, Murtaugh MP. NF-κB-mediated expression of iNOS promotes epithelial defense against infection by Cryptosporidium parvum in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2006; 290:G164–G174.27. Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Curr Drug Targets Inflamm Allergy. 2005; 4:471–479.
Article28. Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000; 109:150–158.
Article29. Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth. 2001; 87:3–11.
Article30. Yum HW, Zhong X, Park J, Na HK, Kim N, Lee HS, Surh YJ. Oligonol inhibits dextran sulfate sodium-induced colitis and colonic adenoma formation in mice. Antioxid Redox Signal. 2013; 19:102–114.
Article31. Noh JS, Kim HY, Park CH, Fujii H, Yokozawa T. Hypolipidaemic and antioxidative effects of oligonol, a low-molecular-weight polyphenol derived from lychee fruit, on renal damage in type 2 diabetic mice. Br J Nutr. 2010; 104:1120–1128.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Neuro-Protective Effect of the Methanolic Extract of Perilla frutescens var. japonica and Rosmarinic Acid against H2O2-Induced Oxidative Stress in C6 Glial Cells
- Protective effect of Cordyceps militaris against hydrogen peroxide-induced oxidative stress in vitro
- Acer okamotoanum Inhibit the Hydrogen Peroxide-Induced Oxidative Stress in C6 Glial Cells
- Role of Morphine in the Glutamate-Induced Oxidative Damage of C6 Glial Cells
- Protective Effect of Nitric Oxide Against Lipopolysaccharide-induced Cytotoxicity in C6-glial Cell