Nutr Res Pract.
2015 Apr;9(2):111-116. 10.4162/nrp.2015.9.2.111.
Ethanol extract of Innotus obliquus (Chaga mushroom) induces G1 cell cycle arrest in HT-29 human colon cancer cells
- Affiliations
-
- 1Department of Food Science and Nutrition, Dongseo University, Busan 617-716, Korea.
- 2Research Institute, Adbiotech Co. Ltd., Gangwon 200-957, Korea.
- 3Department of Technology and Home Economics Education, Kongju National University, 56 Kongjudaehak-ro, Chungnam 314-701, Korea. shkim@kongju.ac.kr
- KMID: 2313817
- DOI: http://doi.org/10.4162/nrp.2015.9.2.111
Abstract
- BACKGROUND/OBJECTIVES
Inonotus obliquus (I. obliquus, Chaga mushroom) has long been used as a folk medicine to treat cancer. In the present study, we examined whether or not ethanol extract of I. obliquus (EEIO) inhibits cell cycle progression in HT-29 human colon cancer cells, in addition to its mechanism of action.
MATERIALS/METHODS
To examine the effects of Inonotus obliquus on the cell cycle progression and the molecular mechanism in colon cancer cells, HT-29 human colon cancer cells were cultured in the presence of 2.5 - 10 microg/mL of EEIO, and analyzed the cell cycle arrest by flow cytometry and the cell cycle controlling protein expression by Western blotting.
RESULTS
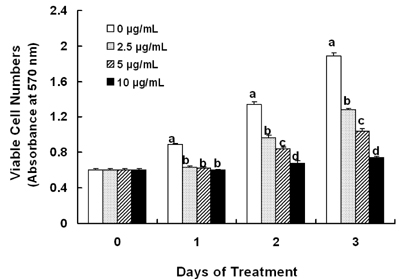
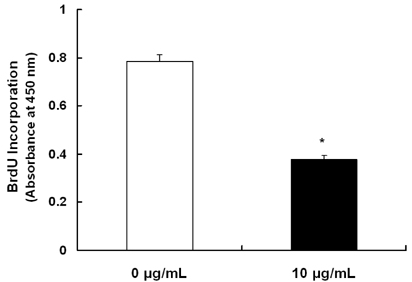
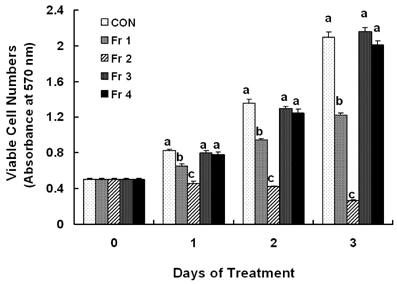
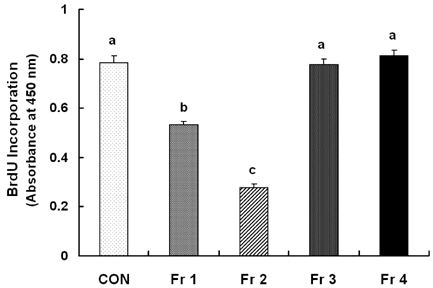
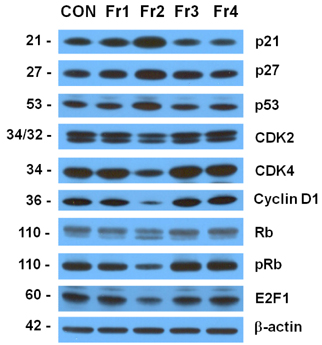
Treatment cells with 2.5 - 10 microg/mL of EEIO reduced viable HT-29 cell numbers and DNA synthesis, increased the percentage of cells in G1 phase, decreased protein expression of CDK2, CDK4, and cyclin D1, increased expression of p21, p27, and p53, and inhibited phosphorylation of Rb and E2F1 expression. Among I. obliquus fractions, fraction 2 (fractionated by dichloromethane from EEIO) showed the same effect as EEIO treatment on cell proliferation and cell cycle-related protein levels.
CONCLUSIONS
These results demonstrate that fraction 2 is the major fraction that induces G1 arrest and inhibits cell proliferation, suggesting I. obliquus could be used as a natural anti-cancer ingredient in the food and/or pharmaceutical industry.
Keyword
MeSH Terms
Figure
Reference
-
1. Taji S, Yamada T, In Y, Wada SI, Usami Y, Sakuma K, Tanaka R. Three new lanostane triterpenoids from Inonotus obliquus. Helv Chim Acta. 2007; 90:2047–2057.2. Saar M. Fungi in khanty folk medicine. J Ethnopharmacol. 1991; 31:175–179.
Article3. Zhao F, Piao H, Han C. Studies on anti-mutation active constituents of the Fuscoporia oblique. J Med Sci Yanbian Univ. 2004; 27:250–252.4. Chen H, Xu X, Zhu Y. Optimization of hydroxyl radical scavenging activity of exo-polysaccharides from Inonotus obliquus in submerged fermentation using response surface methodology. J Microbiol Biotechnol. 2010; 20:835–843.5. Choi SY, Hur SJ, An CS, Jeon YH, Jeoung YJ, Bak JP, Lim BO. Anti-inflammatory effects of Inonotus obliquus in colitis induced by dextran sodium sulfate. J Biomed Biotechnol. 2010; 2010:943516.6. Kahlos K, Kangas L, Hiltunen R. Antitumor activity of some compounds and fractions from an n-hexane extract of Inonotus obliquus in vitro. Acta Pharm Fennica. 1987; 96:33–40.7. Ichimura T, Watanabe O, Maruyama S. Inhibition of HIV-1 protease by water-soluble lignin-like substance from an edible mushroom, Fuscoporia obliqua. Biosci Biotechnol Biochem. 1998; 62:575–577.
Article8. Nakata T, Yamada T, Taji S, Ohishi H, Wada S, Tokuda H, Sakuma K, Tanaka R. Structure determination of inonotsuoxides A and B and in vivo anti-tumor promoting activity of inotodiol from the sclerotia of Inonotus obliquus. Bioorg Med Chem. 2007; 15:257–264.
Article9. Shibnev VA, Mishin DV, Garaev TM, Finogenova NP, Botikov AG, Deryabin PG. Antiviral activity of Inonotus obliquus fungus extract towards infection caused by hepatitis C virus in cell cultures. Bull Exp Biol Med. 2011; 151:612–614.
Article10. Song FQ, Liu Y, Kong XS, Chang W, Song G. Progress on understanding the anticancer mechanisms of medicinal mushroom: inonotus obliquus. Asian Pac J Cancer Prev. 2013; 14:1571–1578.
Article11. Lee SH, Hwang HS, Yun JW. Antitumor activity of water extract of a mushroom, Inonotus obliquus, against HT-29 human colon cancer cells. Phytother Res. 2009; 23:1784–1789.
Article12. Lee IK, Kim YS, Jang YW, Jung JY, Yun BS. New antioxidant polyphenols from the medicinal mushroom Inonotus obliquus. Bioorg Med Chem Lett. 2007; 17:6678–6681.
Article13. Park YM, Won JH, Kim YH, Choi JW, Park HJ, Lee KT. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J Ethnopharmacol. 2005; 101:120–128.
Article14. Nakajima Y, Sato Y, Konishi T. Antioxidant small phenolic ingredients in Inonotus obliquus (persoon) Pilat (Chaga). Chem Pharm Bull (Tokyo). 2007; 55:1222–1226.
Article15. Youn MJ, Kim JK, Park SY, Kim Y, Kim SJ, Lee JS, Chai KY, Kim HJ, Cui MX, So HS, Kim KY, Park R. Chaga mushroom (Inonotus obliquus) induces G0/G1 arrest and apoptosis in human hepatoma HepG2 cells. World J Gastroenterol. 2008; 14:511–517.
Article16. Hyun KW, Jeong SC, Lee DH, Park JS, Lee JS. Isolation and characterization of a novel platelet aggregation inhibitory peptide from the medicinal mushroom, Inonotus obliquus. Peptides. 2006; 27:1173–1178.
Article17. Cui Y, Kim DS, Park KC. Antioxidant effect of Inonotus obliquus. J Ethnopharmacol. 2005; 96:79–85.
Article18. Ma L, Chen H, Dong P, Lu X. Anti-inflammatory and anticancer activities of extracts and compounds from the mushroom Inonotus obliquus. Food Chem. 2013; 139:503–508.
Article19. Senderowicz AM. Novel direct and indirect cyclin-dependent kinase modulators for the prevention and treatment of human neoplasms. Cancer Chemother Pharmacol. 2003; 52:Suppl 1. S61–S73.
Article20. Swanton C. Cell-cycle targeted therapies. Lancet Oncol. 2004; 5:27–36.
Article21. Sherr CJ. Cancer cell cycles. Science. 1996; 274:1672–1677.
Article22. Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999; 39:295–312.
Article23. Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003; 36:131–149.
Article24. Hall M, Peters G. Genetic alterations of cyclins, cyclin-dependent kinases, and Cdk inhibitors in human cancer. Adv Cancer Res. 1996; 68:67–108.
Article25. Kim EJ, Holthuizen PE, Park HS, Ha YL, Jung KC, Park JH. Trans-10,cis-12-conjugated linoleic acid inhibits Caco-2 colon cancer cell growth. Am J Physiol Gastrointest Liver Physiol. 2002; 283:G357–G367.
Article26. Cho HJ, Kim EJ, Lim SS, Kim MK, Sung MK, Kim JS, Park JH. Trans-10,cis-12, not cis-9,trans-11, conjugated linoleic acid inhibits G1-S progression in HT-29 human colon cancer cells. J Nutr. 2006; 136:893–898.
Article27. Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003; 284:G996–G1005.28. Kim EJ, Lim SS, Park SY, Shin HK, Kim JS, Park JH. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem Toxicol. 2008; 46:3651–3658.
Article29. Handa N, Yamada T, Tanaka R. Four new lanostane-type triterpenoids from Inonotus obliquus. Phytochem Lett. 2012; 5:480–485.
Article30. Song Y, Hui J, Kou W, Xin R, Jia F, Wang N, Hu F, Zhang H, Liu H. Identification of Inonotus obliquus and analysis of antioxidation and antitumor activities of polysaccharides. Curr Microbiol. 2008; 57:454–462.
Article31. Sun Y, Yin T, Chen XH, Zhang G, Curtis RB, Lu ZH, Jiang JH. In vitro antitumor activity and structure characterization of ethanol extracts from wild and cultivated Chaga medicinal mushroom, Inonotus obliquus (Pers.:Fr.) Pilát (Aphyllophoromycetideae). Int J Med Mushrooms. 2011; 13:121–130.
Article32. Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993; 366:701–704.
Article33. Gartel AL, Tyner AL. Transcriptional regulation of the p21(WAF1/CIP1) gene. Exp Cell Res. 1999; 246:280–289.34. Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008; 8:253–267.
Article35. Wu CL, Zukerberg LR, Ngwu C, Harlow E, Lees JA. In vivo association of E2F and DP family proteins. Mol Cell Biol. 1995; 15:2536–2546.
Article36. Das SK, Hashimoto T, Shimizu K, Yoshida T, Sakai T, Sowa Y, Komoto A, Kanazawa K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21 WAF1/Cip1. Biochim Biophys Acta. 2005; 1726:328–335.
Article37. Bartkova J, Grøn B, Dabelsteen E, Bartek J. Cell-cycle regulatory proteins in human wound healing. Arch Oral Biol. 2003; 48:125–132.
Article38. Korenjak M, Brehm A. E2F-Rb complexes regulating transcription of genes important for differentiation and development. Curr Opin Genet Dev. 2005; 15:520–527.
Article39. Youn MJ, Kim JK, Park SY, Kim Y, Park C, Kim ES, Park KI, So HS, Park R. Potential anticancer properties of the water extract of Inonotus [corrected] obliquus by induction of apoptosis in melanoma B16-F10 cells. J Ethnopharmacol. 2009; 121:221–228.
Article40. Chung MJ, Chung CK, Jeong Y, Ham SS. Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing Sarcoma-180 cells. Nutr Res Pract. 2010; 4:177–182.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anticancer activity of subfractions containing pure compounds of Chaga mushroom (Inonotus obliquus) extract in human cancer cells and in Balbc/c mice bearing Sarcoma-180 cells
- Immunomodulatory Activity of the Water Extract from Medicinal Mushroom Inonotus obliquus
- Effect of Inonotus Obliques Extracts on Proliferation and Caspase-3 Activity in Human Castro-Intestinal Cancer Cell Lines
- The Improvement of Chaga Mushroom (Inonotus Obliquus) Extract Supplementation on the Blood Glucose and Cellular DNA Damage in Streptozotocin-Induced Diabetic Rats
- Inhibition of c-Yes Induces Differentiation of HT-29 Human Colon Cancer Stem Cells through Midbody Elongation