Nutr Res Pract.
2015 Feb;9(1):22-29. 10.4162/nrp.2015.9.1.22.
Anti-diabetic effect of purple corn extract on C57BL/KsJ db/db mice
- Affiliations
-
- 1College of Food Science and Engineering, Liaoning Medical University, Jinzhou 121000, China.
- 2Institute of Natural Medicine, Hallym University Medical School, Gangwon 200-702, Korea. limss@hallym.ac.kr
- 3Department of Food Science and Nutrition and Center for Aging and HealthCare, Hallym University, 1 Hallymdaehak-gil, Chuncheon, Gangwon 200-702, Korea.
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Hallym University, Gangwon 200-702, Korea.
- 5Department of Biochemistry, School of Medicine, Hallym University, Gangwon 200-702, Korea.
- KMID: 2313805
- DOI: http://doi.org/10.4162/nrp.2015.9.1.22
Abstract
- BACKGROUND/OBJECTIVES
Recently, anthocyanins have been reported to have various biological activities. Furthermore, anthocyanin-rich purple corn extract (PCE) ameliorated insulin resistance and reduced diabetes-associated mesanginal fibrosis and inflammation, suggesting that it may have benefits for the prevention of diabetes and diabetes complications. In this study, we determined the anthocyanins and non-anthocyanin component of PCE by HPLC-ESI-MS and investigated its anti-diabetic activity and mechanisms using C57BL/KsJ db/db mice.
MATERIALS/METHODS
The db/db mice were divided into four groups: diabetic control group (DC), 10 or 50 mg/kg PCE (PCE 10 or PCE 50), or 10 mg/kg pinitol (pinitol 10) and treated with drugs once per day for 8 weeks. During the experiment, body weight and blood glucose levels were measured every week. At the end of treatment, we measured several diabetic parameters.
RESULTS
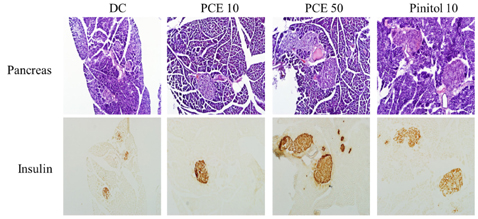
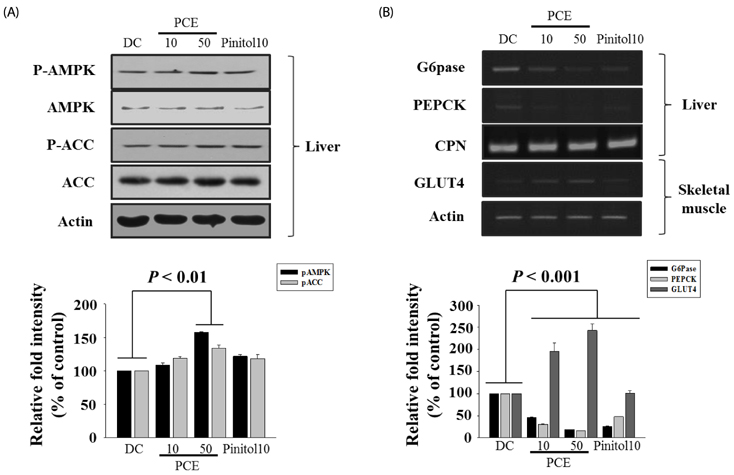
Compared to the DC group, Fasting blood glucose levels were 68% lower in PCE 50 group and 51% lower in the pinitol 10 group. Furthermore, the PCE 50 group showed 2- fold increased C-peptide and adiponectin levels and 20% decreased HbA1c levels, than in the DC group. In pancreatic islets morphology, the PCE- or pinitol-treated mice showed significant prevention of pancreatic beta-cell damage and higher insulin content. Microarray analyses results indicating that gene and protein expressions associated with glycolysis and fatty acid metabolism in liver and fat tissues. In addition, purple corn extract increased the phosphorylation of AMP-activated protein kinase (AMPK) and decreased phosphoenolpyruvate carboxykinase (PEPCK), glucose 6-phosphatase (G6pase) genes in liver, and also increased glucose transporter 4 (GLUT4) expressions in skeletal muscle.
CONCLUSIONS
Our results suggested that PCE exerted anti-diabetic effects through protection of pancreatic beta-cells, increase of insulin secretion and AMPK activation in the liver of C57BL/KsJ db/db mice.
Keyword
MeSH Terms
-
Adiponectin
AMP-Activated Protein Kinases
Animals
Anthocyanins
Blood Glucose
Body Weight
C-Peptide
Diabetes Complications
Fasting
Fibrosis
Glucose Transport Proteins, Facilitative
Glucose-6-Phosphatase
Glycolysis
Inflammation
Insulin
Insulin Resistance
Islets of Langerhans
Liver
Metabolism
Mice*
Muscle, Skeletal
Phosphoenolpyruvate
Phosphorylation
Zea mays*
AMP-Activated Protein Kinases
Adiponectin
Anthocyanins
Blood Glucose
C-Peptide
Glucose Transport Proteins, Facilitative
Glucose-6-Phosphatase
Insulin
Phosphoenolpyruvate
Figure
Reference
-
1. Vinik AI, Vinik E. Prevention of the complications of diabetes. Am J Manag Care. 2003; 3:S63–S80.2. Goldfrank L, Lewin N, Flomenbaum N, Howland MA. The pernicious panacea: herbal medicine. Hosp Physician. 1982; 10:64–69.3. Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989; 8:553–564.
Article4. Jing P, Giusti MM. Characterization of anthocyanin-rich waste from purple corncobs (Zea mays L.) and its application to color milk. J Agric Food Chem. 2005; 22:8775–8781.
Article5. Fossen T, Slimestad R, Andersen OM. Anthocyanins from maize (Zea mays) and reed canarygrass (Phalaris arundinacea). J Agric Food Chem. 2001; 5:2318–2321.
Article6. Hagiwara A, Miyashita K, Nakanishi T, Sano M, Tamano S, Kadota T, Koda T, Nakamura M, Imaida K, Ito N, Shirai T. Pronounced inhibition by a natural anthocyanin, purple corn color, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-associated colorectal carcinogenesis in male F344 rats pretreated with 1,2-dimethylhydrazine. Cancer Lett. 2001; 1:17–25.
Article7. Tsuda T, Horio F, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside increases ex vivo oxidation resistance of serum in rats. Lipids. 1998; 6:583–588.8. Tsuda T, Shiga K, Ohshima K, Kawakishi S, Osawa T. Inhibition of lipid peroxidation and the active oxygen radical scavenging effect of anthocyanin pigments isolated from Phaseolus vulgaris L. Biochem Pharmacol. 1996; 7:1033–1039.
Article9. Yoshimoto M, Okuno S, Yoshinaga M, Yamakawa O, Yamaguchi M, Yamada J. Antimutagenicity of sweetpotato (Ipomoea batatas) roots. Biosci Biotechnol Biochem. 1999; 3:537–541.
Article10. Li J, Lim SS, Lee JY, Kim JK, Kang SW, Kim JL, Kang YH. Purple corn anthocyanins dampened high-glucose-induced mesangial fibrosis and inflammation: possible renoprotective role in diabetic nephropathy. J Nutr Biochem. 2012; 4:320–331.
Article11. Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003; 7:2125–2130.12. Trinder P. Determination of blood glucose using an oxidaseperoxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969; 2:158–161.
Article13. Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol. 2002; 90:3G–10G.
Article14. Liu F, Kim J, Li Y, Liu X, Li J, Chen X. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J Nutr. 2001; 9:2242–2247.
Article15. Guo H, Guo J, Jiang X, Li Z, Ling W. Cyanidin-3-O-b-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food chem Toxicol. 2012; 9:3040–3047.16. Zhao X, Corrales M, Zhang C, Hu X, Ma Y, Tauscher B. Composition and thermal stability of anthocyanins from chinese purple corn (Zea mays L.). J Agric Food Chem. 2008; 22:10761–10766.
Article17. Libby P, Plutzky J. Diabetic macrovascular disease: the glucose paradox? Circulation. 2002; 22:2760–2763.18. Nishina PM, Naggert JK, Verstuyft J, Paigen B. Atherosclerosis in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism. 1994; 5:554–558.
Article19. Narayanan CR, Joshi DD, Mudjumdar AM, Dhekne VV. Pinitol, a new anti-diabetic compound from the leaves of Bougainvillea spectabilis. Curr Sci. 1987; 56:139–141.20. Hong S, Heo J, Kim J, Kwon S, Yeo K, Bakowska-Barczak A, Kolodziejczyk P, Ryu O, Choi M, Kang Y, Lim S, Suh H, Huh S, Lee J. Antidiabetic and Beta Cell-Protection Activities of Purple Corn Anthocyanins. Biomol Ther (Seoul). 2013; 21:284–289.
Article21. Alberti KG, Zimmet PZ. New diagnostic criteria and classification of diabetes--again? Diabet Med. 1998; 7:535–536.
Article22. Scott DK, O'Doherty RM, Stafford JM, Newgard CB, Granner DK. The repression of hormone-activated PEPCK gene expression by glucose is insulin-independent but requires glucose metabolism. J Biol Chem. 1998; 37:24145–24151.
Article23. Bressler R, Brendel K. The role of carnitine and carnitine acyltransferase in biological acetylations and fatty acid synthesis. J Biol Chem. 1966; 17:4092–4097.
Article24. Kelly DP, Gordon JI, Alpers R, Strauss AW. The tissue-specific expression and developmental regulation of two nuclear genes encoding rat mitochondrial proteins. Medium chain acyl-CoA dehydrogenase and mitochondrial malate dehydrogenase. J Biol Chem. 1989; 32:18921–18925.25. Ling B, Aziz C, Alcorn J. Systematic Evaluation of Key L-Carnitine Homeostasis Mechanisms during Postnatal Development in Rat. Nutr Metab (Lond). 2012; 1:66.
Article26. Nemali MR, Usuda N, Reddy MK, Oyasu K, Hashimoto T, Osumi T, Rao MS, Reddy JK. Comparison of constitutive and inducible levels of expression of peroxisomal beta-oxidation and catalase genes in liver and extrahepatic tissues of rat. Cancer Res. 1988; 18:5316–5324.27. Tsuchida T, Fukuda S, Aoyama H, Taniuchi N, Ishihara T, Ohashi N, Sato H, Wakimoto K, Shiotani M, Oku A. MGAT2 deficiency ameliorates high-fat diet-induced obesity and insulin resistance by inhibiting intestinal fat absorption in mice. Lipids Health Dis. 2012; 11:75.
Article28. Jiang Z, Michal JJ, Chen J, Daniels TF, Kunej T, Garcia MD, Gaskins CT, Busboom JR, Alexander LJ, Wright RW Jr, Macneil MD. Discovery of novel genetic networks associated with 19 economically important traits in beef cattle. Int J Biol Sci. 2009; 6:528–542.
Article29. Wu XL, Macneil MD, De S, Xiao QJ, Michal JJ, Gaskins CT, Reeves JJ, Busboom JR, Wright RW Jr, Jiang Z. Evaluation of candidate gene effects for beef backfat via Bayesian model selection. Genetica. 2005; 1:103–113.
Article30. Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004; 23:5479–5487.31. Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007; 3:328–341.
Article32. Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999; 1:22–25.
Article33. Rutter GA, Da Silva Xavier G, Leclerc I. Roles of 5'-AMP-activated protein kinase (AMPK) in mammalian glucose homoeostasis. Biochem J. 2003; 1:1–16.
Article34. Velasco G, Geelen MJ, Guzmán M. Control of hepatic fatty acid oxidation by 5'-AMP-activated protein kinase involves a malonyl-CoA-dependent and a malonyl-CoA-independent mechanism. Arch Biochem Biophys. 1997; 2:169–175.
Article35. Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997; 1:E1107–E1112.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice
- Silk fibroin hydrolysate exerts an anti-diabetic effect by increasing pancreatic beta cell mass in C57BL/KsJ-db/db mice
- Gynura procumbens extract improves insulin sensitivity and suppresses hepatic gluconeogenesis in C57BL/KsJ-db/db mice
- Effect of beta-glucan from Aureobasidium on dermal wound healing in diabetic C57BL/KsJ-db/db mouse model
- Effects of Inonotus Obliqua Extract on Blood Glucose Levels in Genetically Diabetic Mice