Nutr Res Pract.
2015 Feb;9(1):3-10. 10.4162/nrp.2015.9.1.3.
Intestinal anti-inflammatory activity of Sasa quelpaertensis leaf extract by suppressing lipopolysaccharide-stimulated inflammatory mediators in intestinal epithelial Caco-2 cells co-cultured with RAW 264.7 macrophage cells
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, 52, Ewhayeodae-gil, Seodaemun-gu, Seoul 120-750, Korea. yuri.kim@ewha.ac.kr
- 2Jeju Sasa Industry Development Agency, Jeju National University, Jeju 690-756, Korea.
- 3Department of Biology, Jeju National University, Jeju 690-756, Korea.
- KMID: 2313802
- DOI: http://doi.org/10.4162/nrp.2015.9.1.3
Abstract
- BACKGROUND/OBJECTIVES
Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, involves chronic inflammation of the gastrointestinal tract. Previously, Sasa quelpaertensis leaves have been shown to mediate anti-inflammation and anti-cancer effects, although it remains unclear whether Sasa leaves are able to attenuate inflammation-related intestinal diseases. Therefore, the aim of this study was to investigate the anti-inflammatory effects of Sasa quelpaertensis leaf extract (SQE) using an in vitro co-culture model of the intestinal epithelial environment.
MATERIALS/METHODS
An in vitro co-culture system was established that consisted of intestinal epithelial Caco-2 cells and RAW 264.7 macrophages. Treatment with lipopolysaccharide (LPS) was used to induce inflammation.
RESULTS
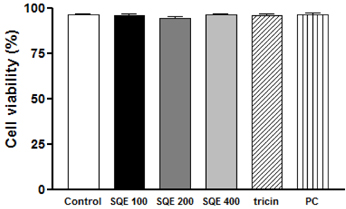
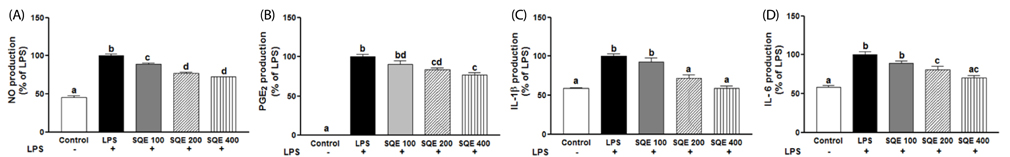
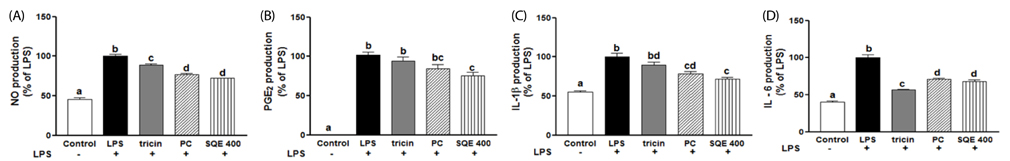
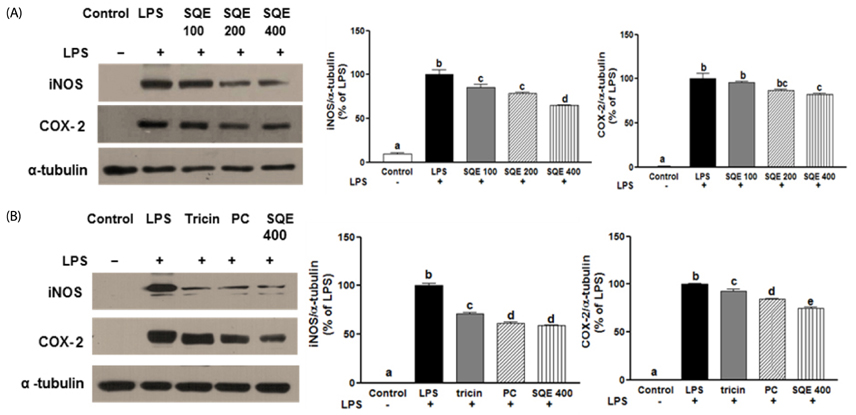
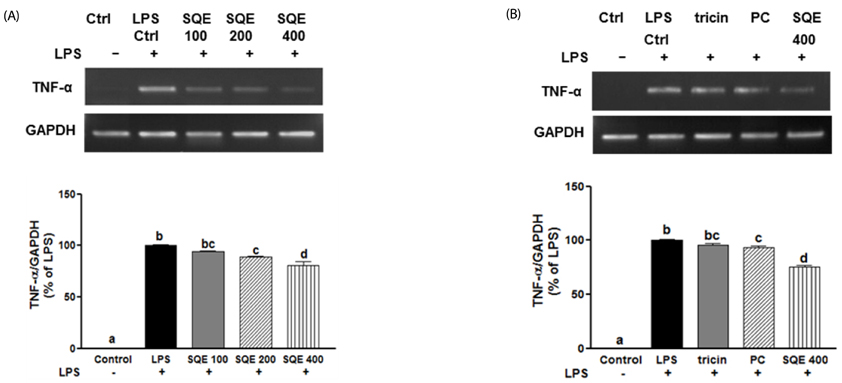
Treatment with SQE significantly suppressed the secretion of LPS-induced nitric oxide (NO), prostaglandin E2 (PGE2), IL-6, and IL-1beta in co-cultured RAW 264.7 macrophages. In addition, expressions of inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2, and tumor necrosis factor (TNF)-alpha were down-regulated in response to inhibition of IkappaBalpha phosphorylation by SQE. Compared with two bioactive compounds that have previously been identified in SQE, tricin and P-coumaric acid, SQE exhibited the most effective anti-inflammatory properties.
CONCLUSIONS
SQE exhibited intestinal anti-inflammatory activity by inhibiting various inflammatory mediators mediated through nuclear transcription factor kappa-B (NF-kB) activation. Thus, SQE has the potential to ameliorate inflammation-related diseases, including IBD, by limiting excessive production of pro-inflammatory mediators.
MeSH Terms
-
Caco-2 Cells*
Coculture Techniques
Colitis, Ulcerative
Crohn Disease
Dinoprostone
Gastrointestinal Tract
Humans
Inflammation
Inflammatory Bowel Diseases
Interleukin-6
Intestinal Diseases
Macrophages*
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphorylation
Prostaglandin-Endoperoxide Synthases
Sasa*
Transcription Factors
Tumor Necrosis Factor-alpha
Dinoprostone
Interleukin-6
Nitric Oxide
Nitric Oxide Synthase Type II
Prostaglandin-Endoperoxide Synthases
Transcription Factors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Arai F, Takahashi T, Furukawa K, Matsushima K, Asakura H. Mucosal expression of interleukin-6 and interleukin-8 messenger RNA in ulcerative colitis and in Crohn's disease. Dig Dis Sci. 1998; 43:2071–2079.2. Chung HL, Yue GG, To KF, Su YL, Huang Y, Ko WH. Effect of Scutellariae Radix extract on experimental dextran-sulfate sodium-induced colitis in rats. World J Gastroenterol. 2007; 13:5605–5611.
Article3. Loftus EV Jr, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002; 31:1–20.
Article4. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003; 3:521–533.
Article5. Menditto A, Menotti A, Morisi G, Patriarca M, Spagnolo A. Serum ascorbic acid levels in men aged 55-75 years: association to selected social factors and biochemical parameters. Arch Gerontol Geriatr. 1992; 15:Suppl 1. 257–265.
Article6. Nishitani Y, Tanoue T, Yamada K, Ishida T, Yoshida M, Azuma T, Mizuno M. Lactococcus lactis subsp. cremoris FC alleviates symptoms of colitis induced by dextran sulfate sodium in mice. Int Immunopharmacol. 2009; 9:1444–1451.
Article7. Bode H, Schmitz H, Fromm M, Scholz P, Riecken EO, Schulzke JD. IL-1beta and TNF-alpha, but not IFN-alpha, IFN-gamma, IL-6 or IL-8, are secretory mediators in human distal colon. Cytokine. 1998; 10:457–465.
Article8. Brozek W, Bises G, Fabjani G, Cross HS, Peterlik M. Clone-specific expression, transcriptional regulation, and action of interleukin-6 in human colon carcinoma cells. BMC Cancer. 2008; 8:13.
Article9. Edwards MR, Bartlett NW, Clarke D, Birrell M, Belvisi M, Johnston SL. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther. 2009; 121:1–13.
Article10. Wong ET, Tergaonkar V. Roles of NF-kappaB in health and disease: mechanisms and therapeutic potential. Clin Sci (Lond). 2009; 116:451–465.
Article11. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990; 98:694–702.
Article12. Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993; 75:253–261.
Article13. Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993; 75:263–274.
Article14. Ohta N, Hiroi T, Kweon MN, Kinoshita N, Jang MH, Mashimo T, Miyazaki J, Kiyono H. IL-15-dependent activation-induced cell death-resistant Th1 type CD8 alpha beta + NK1.1 + T cells for the development of small intestinal inflammation. J Immunol. 2002; 169:460–468.
Article15. Farombi EO, Adedara IA, Ajayi BO, Ayepola OR, Egbeme EE. Kolaviron, a natural antioxidant and anti-inflammatory phytochemical prevents dextran sulphate sodium-induced colitis in rats. Basic Clin Pharmacol Toxicol. 2013; 113:49–55.
Article16. Okabe S, Takeuchi K, Takagi K, Shibata M. Stimulatory effect of the water extract of bamboo grass (Folin solution) on gastric acid secretion in pylorus-ligated rats. Jpn J Pharmacol. 1975; 25:608–609.
Article17. Choi YJ, Lim HS, Choi JS, Shin SY, Bae JY, Kang SW, Kang IJ, Kang YH. Blockade of chronic high glucose-induced endothelial apoptosis by Sasa borealis bamboo extract. Exp Biol Med (Maywood). 2008; 233:580–591.
Article18. Ren M, Reilly RT, Sacchi N. Sasa health exerts a protective effect on Her2/NeuN mammary tumorigenesis. Anticancer Res. 2004; 24:2879–2884.19. Kang SI, Shin HS, Kim HM, Hong YS, Yoon SA, Kang SW, Kim JH, Ko HC, Kim SJ. Anti-obesity properties of a Sasa quelpaertensis extract in high-fat diet-induced obese mice. Biosci Biotechnol Biochem. 2012; 76:755–761.
Article20. Kim K, Lim JY, Min S, Lim Y, Ko H, Kim S, Kim Y. Sasa quelpaertensis leaf extract suppresses dextran sulfate sodium (DSS)-induced colitis im mice. Nutr Res. Forthcoming 2014.21. Tanoue T, Nishitani Y, Kanazawa K, Hashimoto T, Mizuno M. In vitro model to estimate gut inflammation using co-cultured Caco-2 and RAW264.7 cells. Biochem Biophys Res Commun. 2008; 374:565–569.
Article22. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem. 1976; 72:248–254.
Article23. Wilson KT, Ramanujam KS, Mobley HL, Musselman RF, James SP, Meltzer SJ. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology. 1996; 111:1524–1533.
Article24. Choi EM, Hwang JK. Effects of Morus alba leaf extract on the production of nitric oxide, prostaglandin E2 and cytokines in RAW264.7 macrophages. Fitoterapia. 2005; 76:608–613.
Article25. Shapira L, Soskolne WA, Houri Y, Barak V, Halabi A, Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect Immun. 1996; 64:825–828.
Article26. Jeoung BR, Lee KD, Na CS, Kim YE, Kim B, Kim YR. Ganghwaljetongyeum, an anti-arthritic remedy, attenuates synoviocyte proliferation and reduces the production of proinflammatory mediators in macrophages: the therapeutic effect of GHJTY on rheumatoid arthritis. BMC Complement Altern Med. 2013; 13:47.
Article27. Lee H, Bae S, Choi BW, Yoon Y. WNT/beta-catenin pathway is modulated in asthma patients and LPS-stimulated RAW264.7 macrophage cell line. Immunopharmacol Immunotoxicol. 2012; 34:56–65.
Article28. Okada Y, Tsuzuki Y, Narimatsu K, Sato H, Ueda T, Hozumi H, Sato S, Hokari R, Kurihara C, Komoto S, Watanabe C, Tomita K, Kawaguchi A, Nagao S, Miura S. 1,4-Dihydroxy-2-naphthoic acid from Propionibacterium freudenreichii reduces inflammation in interleukin-10-deficient mice with colitis by suppressing macrophage-derived proinflammatory cytokines. J Leukoc Biol. 2013; 94:473–480.
Article29. MacDermott RP. Chemokines in the inflammatory bowel diseases. J Clin Immunol. 1999; 19:266–272.30. Ogata H, Hibi T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr Pharm Des. 2003; 9:1107–1113.
Article31. Feagan BG, Reinisch W, Rutgeerts P, Sandborn WJ, Yan S, Eisenberg D, Bala M, Johanns J, Olson A, Hanauer SB. The effects of infliximab therapy on health-related quality of life in ulcerative colitis patients. Am J Gastroenterol. 2007; 102:794–802.
Article32. Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003; 17:1411–1421.
Article33. Appleby SB, Ristimäki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994; 302(Pt 3):723–727.
Article34. Beg AA, Finco TS, Nantermet PV, Baldwin AS Jr. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993; 13:3301–3310.
Article35. Park OJ, Surh YJ. Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett. 2004; 150:43–56.
Article36. Bai SK, Lee SJ, Na HJ, Ha KS, Han JA, Lee H, Kwon YG, Chung CK, Kim YM. beta-Carotene inhibits inflammatory gene expression in lipopolysaccharide-stimulated macrophages by suppressing redox-based NF-kappaB activation. Exp Mol Med. 2005; 37:323–334.
Article37. Liu H, Sidiropoulos P, Song G, Pagliari LJ, Birrer MJ, Stein B, Anrather J, Pope RM. TNF-alpha gene expression in macrophages: regulation by NF-kappa B is independent of c-Jun or C/EBP beta. J Immunol. 2000; 164:4277–4285.
Article38. Rosillo MA, Sanchez-Hidalgo M, Cárdeno A, de la Lastra CA. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn's disease. Biochem Pharmacol. 2011; 82:737–745.
Article39. Chu X, Ci X, He J, Jiang L, Wei M, Cao Q, Guan M, Xie X, Deng X, He J. Effects of a natural prolyl oligopeptidase inhibitor, rosmarinic acid, on lipopolysaccharide-induced acute lung injury in mice. Molecules. 2012; 17:3586–3598.
Article40. Otani K, Yanaura S, Yuda Y, Kawaoto H, Kajita T, Hirano F, Osawa F, Inouye S. Histo-chemical studies on the anti-ulcer effect of bamboo grass in rats. Int J Tissue React. 1990; 12:319–332.41. Moon JY, Yang EJ, Kim SS, Kang JY, Kim GO, Lee NH, Hyun CG. Sasa quelpaertensis phenylpropanoid derivative suppresses lipopolysaccharide-induced nitric oxide synthase and cyclo-oxygenase-2 expressions in RAW 264.7 cells. Yakugaku Zasshi. 2011; 131:961–967.
Article42. Zang LY, Cosma G, Gardner H, Shi X, Castranova V, Vallyathan V. Effect of antioxidant protection by P-coumaric acid on low-density lipoprotein cholesterol oxidation. Am J Physiol Cell Physiol. 2000; 279:C954–C960.43. Pragasam SJ, Venkatesan V, Rasool M. Immunomodulatory and anti-inflammatory effect of P-coumaric acid, a common dietary polyphenol on experimental inflammation in rats. Inflammation. 2013; 36:169–176.
Article44. Pérez-Alvarez V, Bobadilla RA, Muriel P. Structure-hepatoprotective activity relationship of 3,4-dihydroxycinnamic acid (caffeic acid) derivatives. J Appl Toxicol. 2001; 21:527–531.
Article45. Shalini V, Bhaskar S, Kumar KS, Mohanlal S, Jayalekshmy A, Helen A. Molecular mechanisms of anti-inflammatory action of the flavonoid, tricin from Njavara rice (Oryza sativa L.) in human peripheral blood mononuclear cells: possible role in the inflammatory signaling. Int Immunopharmacol. 2012; 14:32–38.
Article46. Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003; 78:517S–520S.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of fruit and leaf extracts of Lycium barbarum in lipopolysaccharide-stimulated RAW264.7 cells and animal model
- Anti-inflammatory Effect of Mangosteen (Garcinia mangostana L.) Peel Extract and its Compounds in LPS-induced RAW264.7 Cells
- Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-kappaB and Phosphorylation of JNK in RAW 264.7 Macrophage Cells
- Rhodanthpyrone A and B play an anti-inflammatory role by suppressing the nuclear factor-κB pathway in macrophages
- CXC and CC Chemokine Expression by Intestinal Epithelial Cells in Response to Clostridium difficile Toxin A