Nutr Res Pract.
2014 Dec;8(6):625-631. 10.4162/nrp.2014.8.6.625.
Cholesterol-induced inflammation and macrophage accumulation in adipose tissue is reduced by a low carbohydrate diet in guinea pigs
- Affiliations
-
- 1Department of Nutritional Sciences, University of Connecticut, 3624 Horsebarn Road Ext, U 4017, Storrs, CT 6269, USA. maria-luz.fernandez@uconn.edu
- 2Department of Kinesiology, University of Connecticut, USA.
- KMID: 2313788
- DOI: http://doi.org/10.4162/nrp.2014.8.6.625
Abstract
- BACKGROUND/OBJECTIVES
The main objective of this study was to evaluate the effects of a high cholesterol (HC) dietary challenge on cholesterol tissue accumulation, inflammation, adipocyte differentiation, and macrophage infiltration in guinea pigs. A second objective was to assess whether macronutrient manipulation would reverse these metabolic alterations.
MATERIALS/METHODS
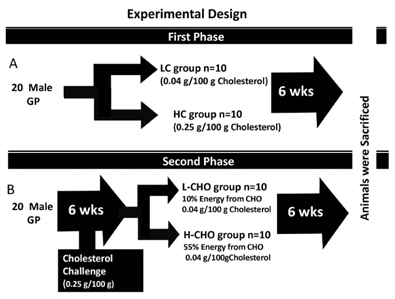
Male Hartley guinea pigs (10/group) were assigned to either low cholesterol (LC) (0.04g/100g) or high cholesterol (HC) (0.25g/100g) diets for six weeks. For the second experiment, 20 guinea pigs were fed the HC diet for six weeks and then assigned to either a low carbohydrate (CHO) diet (L-CHO) (10% energy from CHO) or a high CHO diet (H-CHO) (54% CHO) for an additional six weeks.
RESULTS
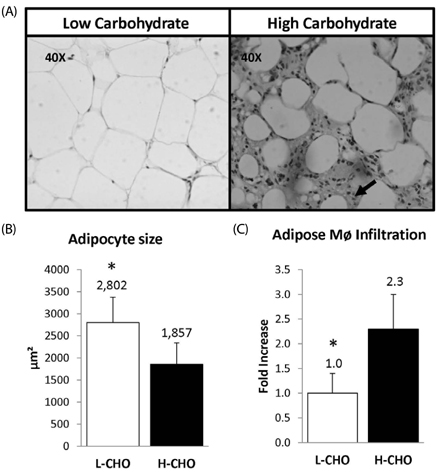
Higher concentrations of total (P < 0.005) and free (P < 0.05) cholesterol were observed in both adipose tissue and aortas of guinea pigs fed the HC compared to those in the LC group. In addition, higher concentrations of pro-inflammatory cytokines in the adipose tissue (P < 0.005) and lower concentrations of anti-inflammatory interleukin (IL)-10 were observed in the HC group (P < 0.05) compared to the LC group. Of particular interest, adipocytes in the HC group were smaller in size (P < 0.05) and showed increased macrophage infiltration compared to the LC group. When compared to the H-CHO group, lower concentrations of cholesterol in both adipose and aortas as well as lower concentrations of inflammatory cytokines in adipose tissue were observed in the L-CHO group (P < 0.05). In addition, guinea pigs fed the L-CHO exhibited larger adipose cells and lower macrophage infiltration compared to the H-CHO group.
CONCLUSIONS
The results of this study strongly suggest that HC induces metabolic dysregulation associated with inflammation in adipose tissue and that L-CHO is more effective than H-CHO in attenuating these detrimental effects.
MeSH Terms
Figure
Reference
-
1. Subramanian S, Chait A. The effect of dietary cholesterol on macrophage accumulation in adipose tissue: implications for systemic inflammation and atherosclerosis. Curr Opin Lipidol. 2009; 20:39–44.
Article2. Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A 3rd, Kirk EA, O'Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008; 28:685–691.
Article3. McGillicuddy FC, Reilly MP, Rader DJ. Adipose modulation of high-density lipoprotein cholesterol: implications for obesity, highdensity lipoprotein metabolism, and cardiovascular disease. Circulation. 2011; 124:1602–1605.
Article4. Krause BR, Hartman AD. Adipose tissue and cholesterol metabolism. J Lipid Res. 1984; 25:97–110.
Article5. Marí M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, García-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006; 4:185–198.
Article6. Schwabe RF, Maher JJ. Lipids in liver disease: looking beyond steatosis. Gastroenterology. 2012; 142:8–11.
Article7. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–867.
Article8. Li H, Li H, Guo H, Liu F. Cholesterol suppresses adipocytic differentiation of mouse adipose-derived stromal cells via PPARgamma2 signaling. Steroids. 2013; 78:454–461.
Article9. Boling CL, Westman EC, Yancy WS Jr. Carbohydrate-restricted diets for obesity and related diseases: an update. Curr Atheroscler Rep. 2009; 11:462–469.
Article10. Leite JO, DeOgburn R, Ratliff J, Su R, Smyth JA, Volek JS, McGrane MM, Dardik A, Fernandez ML. Low-carbohydrate diets reduce lipid accumulation and arterial inflammation in guinea pigs fed a high-cholesterol diet. Atherosclerosis. 2010; 209:442–448.
Article11. Sharman MJ, Fernandez ML, Zern TL, Torres-Gonzalez M, Kraemer WJ, Volek JS. Replacing dietary carbohydrate with protein and fat decreases the concentrations of small LDL and the inflammatory response induced by atherogenic diets in the guinea pig. J Nutr Biochem. 2008; 19:732–738.
Article12. Torres-Gonzalez M, Volek JS, Leite JO, Fraser H, Luz Fernandez M. Carbohydrate restriction reduces lipids and inflammation and prevents atherosclerosis in Guinea pigs. J Atheroscler Thromb. 2008; 15:235–243.
Article13. Wylie-Rosett J, Davis NJ. Low-carbohydrate diets: an update on current research. Curr Diab Rep. 2009; 9:396–404.
Article14. Kim JE, Clark RM, Park Y, Lee J, Fernandez ML. Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr Res Pract. 2012; 6:113–119.
Article15. Carr TP, Andresen CJ, Rudel LL. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin Biochem. 1993; 26:39–42.
Article16. Kim JE, Leite JO, DeOgburn R, Smyth JA, Clark RM, Fernandez ML. A lutein-enriched diet prevents cholesterol accumulation and decreases oxidized LDL and inflammatory cytokines in the aorta of guinea pigs. J Nutr. 2011; 141:1458–1463.
Article17. Torres-Gonzalez M, Shrestha S, Sharman M, Freake HC, Volek JS, Fernandez ML. Carbohydrate restriction alters hepatic cholesterol metabolism in guinea pigs fed a hypercholesterolemic diet. J Nutr. 2007; 137:2219–2223.
Article18. Volek JS, Fernandez ML, Feinman RD, Phinney SD. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog Lipid Res. 2008; 47:307–318.
Article19. Račková L. Cholesterol load of microglia: contribution of membrane architecture changes to neurotoxic power? Arch Biochem Biophys. 2013; 537:91–103.
Article20. Terrand J, Bruban V, Zhou L, Gong W, El Asmar Z, May P, Zurhove K, Haffner P, Philippe C, Woldt E, Matz RL, Gracia C, Metzger D, Auwerx J, Herz J, Boucher P. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J Biol Chem. 2009; 284:381–388.
Article21. Hofmann SM, Zhou L, Perez-Tilve D, Greer T, Grant E, Wancata L, Thomas A, Pfluger PT, Basford JE, Gilham D, Herz J, Tschöp MH, Hui DY. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J Clin Invest. 2007; 117:3271–3282.
Article22. Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996; 98:1575–1584.
Article23. Lowell BB. PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999; 99:239–242.
Article24. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994; 79:1147–1156.
Article25. Shimomura I, Bashmakov Y, Shimano H, Horton JD, Goldstein JL, Brown MS. Cholesterol feeding reduces nuclear forms of sterol regulatory element binding proteins in hamster liver. Proc Natl Acad Sci U S A. 1997; 94:12354–12359.
Article26. Cucuianu M, Coca M, Hâncu N. Reverse cholesterol transport and atherosclerosis. A mini review. Rom J Intern Med. 2007; 45:17–27.27. Zhang Y, McGillicuddy FC, Hinkle CC, O'Neill S, Glick JM, Rothblat GH, Reilly MP. Adipocyte modulation of high-density lipoprotein cholesterol. Circulation. 2010; 121:1347–1355.
Article28. Chung S, Sawyer JK, Gebre AK, Maeda N, Parks JS. Adipose tissue ATP binding cassette transporter A1 contributes to high-density lipoprotein biogenesis in vivo. Circulation. 2011; 124:1663–1672.
Article29. Aronson D, Rayfield EJ. How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc Diabetol. 2002; 1:1.30. Bucala R, Makita Z, Koschinsky T, Cerami A, Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci U S A. 1993; 90:6434–6438.
Article31. Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci U S A. 1994; 91:9441–9445.
Article32. Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988; 318:1315–1321.
Article33. Baranowski M. Biological role of liver X receptors. J Physiol Pharmacol. 2008; 59:Suppl 7. 31–55.34. Leite JO, DeOgburn R, Ratliff JC, Su R, Volek JS, McGrane MM, Dardik A, Fernandez ML. Low-carbohydrate diet disrupts the association between insulin resistance and weight gain. Metabolism. 2009; 58:1116–1122.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of a Low-carbohydrate, High-fat Diet
- The Inhibitory Effect of Quercetin on Adipose Tissue Inflammation in Mice Fed on a High-fat Diet
- Animal Models for Biliary Diseases
- Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet
- Novel Role of Invariant Natural Killer T-cell in Glycemic Control: Regulation by human Adenovirus 36