Nutr Res Pract.
2014 Dec;8(6):613-617. 10.4162/nrp.2014.8.6.613.
Anti-adipogenic effect of mulberry leaf ethanol extract in 3T3-L1 adipocytes
- Affiliations
-
- 1Department of Food and Nutrition and Human Ecology Research Institute, Chonnam National University, Jeonnam 500-757, Korea.
- 2Department of Food and Nutrition, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 130-701, Korea. ylim@khu.ac.kr
- KMID: 2313786
- DOI: http://doi.org/10.4162/nrp.2014.8.6.613
Abstract
- BACKGROUND/OBJECTIVES
Adipogenesis is part of the cell differentiation process in which undifferentiated fibroblasts (pre-adipocytes) become mature adipocytes with the accumulation of lipid droplets and subsequent cell morphological changes. Several transcription factors and food components have been suggested to be involved in adipogenesis. The aim of this study was to determine whether mulberry leaf ethanol extract (MLEE) affects adipogenesis in 3T3-L1 adipocytes.
MATERIALS/METHODS
The 3T3-L1 adipocytes were treated with different doses of MLEE for 8 days starting 2 days post-confluence. Cell viability, fat accumulation, and adipogenesis-related factors including CCAAT-enhancer-binding protein alpha (C/EBPalpha), peroxisome proliferator-activated receptor gamma (PPARgamma), PPARgamma coactivator 1 alpha (PGC-1alpha), fatty acid synthase (FAS), and adiponectin were analyzed.
RESULTS
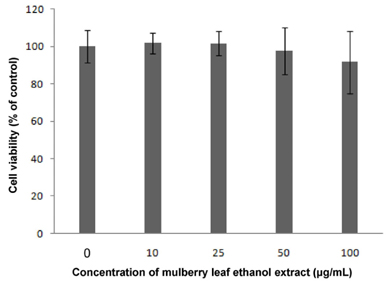
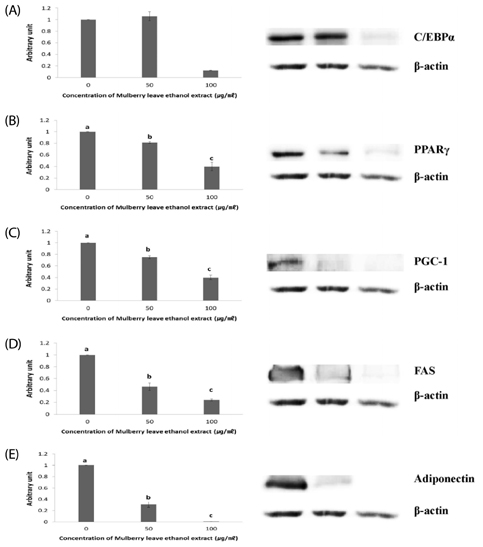
Results showed that MLEE treatments at 10, 25, 50, and 100 microg/ml had no effect on cell morphology and viability. Without evident toxicity, all MLEE treated cells had lower fat accumulation compared with control as shown by lower absorbances of Oil Red O stain. MLEE at 50 and 100 microg/ml significantly reduced protein levels of PPARgamma, PGC-1alpha, FAS, and adiponectin in differentiated adipocytes. Furthermore, protein level of C/EBPalpha was significantly decreased by the treatment of 100 microg/ml MLEE.
CONCLUSION
These results demonstrate that MLEE treatment has an anti-adipogenic effect in differentiated adipocytes without toxicity, suggesting its potential as an anti-obesity therapeutic.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Lipolytic effect of novel extracts from mulberry (
Morus alba ) leaves fermented withCordyceps militaris in the primary adipocytes derived from SD rats
Mi Rim Lee, Ji Eun Kim, Woo Bin Yun, Jun Young Choi, Jin Ju Park, Hye Ryeong Kim, Bo Ram Song, Young Whan Choi, Kyung Mi Kim, Dae Youn Hwang
Lab Anim Res. 2017;33(3):270-279. doi: 10.5625/lar.2017.33.3.270.
Reference
-
1. Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol. 2013; 92:229–236.
Article2. Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009; 20:107–114.
Article3. Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007; 92:1023–1033.
Article4. Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res. 2005; 46:1369–1379.
Article5. Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005; 123:993–999.6. Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ Jr, Liu XS, Lazar MA. PPARgamma and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008; 22:2941–2952.
Article7. Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signalling during adipogenesis. Biochem J. 2003; 376:607–613.
Article8. Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000; 289:950–953.
Article9. Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol. 2006; 26:5827–5837.
Article10. Choi EM, Hwang JK. Effects of Morus alba leaf extract on the production of nitric oxide, prostaglandin E2 and cytokines in RAW264.7 macrophages. Fitoterapia. 2005; 76:608–613.
Article11. Lim HH, Lee SO, Kim SY, Yang SJ, Lim Y. Anti-inflammatory and antiobesity effects of mulberry leaf and fruit extract on high fat diet-induced obesity. Exp Biol Med (Maywood). 2013; 238:1160–1169.
Article12. Lim HH, Yang SJ, Kim Y, Lee M, Lim Y. Combined treatment of mulberry leaf and fruit extract ameliorates obesity-related inflammation and oxidative stress in high fat diet-induced obese mice. J Med Food. 2013; 16:673–680.
Article13. Naowaboot J, Pannangpetch P, Kukongviriyapan V, Prawan A, Kukongviriyapan U, Itharat A. Mulberry leaf extract stimulates glucose uptake and GLUT4 translocation in rat adipocytes. Am J Chin Med. 2012; 40:163–175.
Article14. Tanabe K, Nakamura S, Omagari K, Oku T. Repeated ingestion of the leaf extract from Morus alba reduces insulin resistance in KK-Ay mice. Nutr Res. 2011; 31:848–854.
Article15. Sugimoto M, Arai H, Tamura Y, Murayama T, Khaengkhan P, Nishio T, Ono K, Ariyasu H, Akamizu T, Ueda Y, Kita T, Harada S, Kamei K, Yokode M. Mulberry leaf ameliorates the expression profile of adipocytokines by inhibiting oxidative stress in white adipose tissue in db/db mice. Atherosclerosis. 2009; 204:388–394.
Article16. Lee YJ, Choi DH, Kim EJ, Kim HY, Kwon TO, Kang DG, Lee HS. Hypotensive, hypolipidemic, and vascular protective effects of Morus alba L. in rats fed an atherogenic diet. Am J Chin Med. 2011; 39:39–52.
Article17. Oh KS, Ryu SY, Lee S, Seo HW, Oh BK, Kim YS, Lee BH. Melanin-concentrating hormone-1 receptor antagonism and anti-obesity effects of ethanolic extract from Morus alba leaves in diet-induced obese mice. J Ethnopharmacol. 2009; 122:216–220.
Article18. Peng CH, Liu LK, Chuang CM, Chyau CC, Huang CN, Wang CJ. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J Agric Food Chem. 2011; 59:2663–2671.
Article19. Cowherd RM, Lyle RE, McGehee RE Jr. Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999; 10:3–10.
Article20. Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond). 2005; 29:Suppl 1. S5–S9.21. Kim MY, Park BY, Lee HS, Park EK, Hahm JC, Lee J, Hong Y, Choi S, Park D, Lee H, Yoon M. The anti-angiogenic herbal composition Ob-X inhibits adipose tissue growth in obese mice. Int J Obes (Lond). 2010; 34:820–830.
Article22. Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008; 22:1367–1371.
Article23. Tsuduki T, Nakamura Y, Honma T, Nakagawa K, Kimura T, Ikeda I, Miyazawa T. Intake of 1-deoxynojirimycin suppresses lipid accumulation through activation of the beta-oxidation system in rat liver. J Agric Food Chem. 2009; 57:11024–11029.
Article24. Naowaboot J, Chung CH, Pannangpetch P, Choi R, Kim BH, Lee MY, Kukongviriyapan U. Mulberry leaf extract increases adiponectin in murine 3T3-L1 adipocytes. Nutr Res. 2012; 32:39–44.
Article25. Tang QQ, Grønborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc Natl Acad Sci U S A. 2005; 102:9766–9771.
Article26. Hamm JK, Park BH, Farmer SR. A role for C/EBPbeta in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J Biol Chem. 2001; 276:18464–18471.
Article27. Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem. 1998; 273:30057–30060.
Article28. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999; 4:611–617.
Article29. Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999; 3:151–158.
Article30. Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005; 257:167–175.
Article31. Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005; 26:439–451.
Article32. Pajvani UB, Scherer PE. Adiponectin: systemic contributor to insulin sensitivity. Curr Diab Rep. 2003; 3:207–213.
Article33. Ota T. Chemokine systems link obesity to insulin resistance. Diabetes Metab J. 2013; 37:165–172.
Article34. Körner A, Wabitsch M, Seidel B, Fischer-Posovszky P, Berthold A, Stumvoll M, Blüher M, Kratzsch J, Kiess W. Adiponectin expression in humans is dependent on differentiation of adipocytes and down-regulated by humoral serum components of high molecular weight. Biochem Biophys Res Commun. 2005; 337:540–550.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation
- Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte
- Nelumbo nucifera Leaf Extract Regulates Lipid Metabolism and Differentiation in 3T3-L1 Adipocytes and db/db Mice
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling