Nutr Res Pract.
2014 Oct;8(5):544-549. 10.4162/nrp.2014.8.5.544.
Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet
- Affiliations
-
- 1Department of Smart Foods and Drugs, School of Food and Life Science, Inje University, 197 Inje-ro, Gimhae, Gyungnam 621-749, Korea. fdsnkiji@inje.ac.kr
- 2Food & Nutrition Research Team, Hurom Co., Ltd., 79 Seobu-ro, Gimhae, Gyungnam 621-846, Korea.
- KMID: 2313777
- DOI: http://doi.org/10.4162/nrp.2014.8.5.544
Abstract
- BACKGROUND/OBJECTIVES
Obesity-associated insulin resistance is a strong risk factor for type 2 diabetes mellitus. The aim of this study was to investigate the effect of myricetin on adiposity, insulin resistance, and inflammatory markers in mice with diet-induced insulin resistance.
MATERIALS/METHODS
Five-week-old male C57BL/6J mice were fed a basal diet, a high-fat, high-sucrose (HFHS) diet, or the HFHS diet containing 0.06% myricetin or 0.12% myricetin for 12 weeks after a 1-week adaptation, and body weight and food intake were monitored. After sacrifice, serum lipid profiles, glucose, insulin, adipocyte-derived hormones, and proinflammatory cytokines were measured. The homeostasis model assessment for insulin resistance (HOMA-IR) was determined.
RESULTS
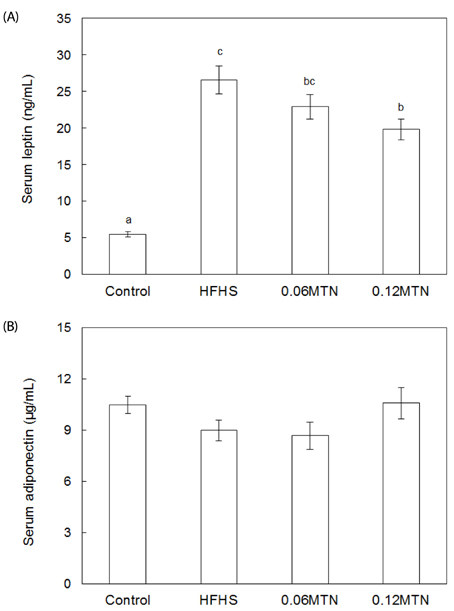
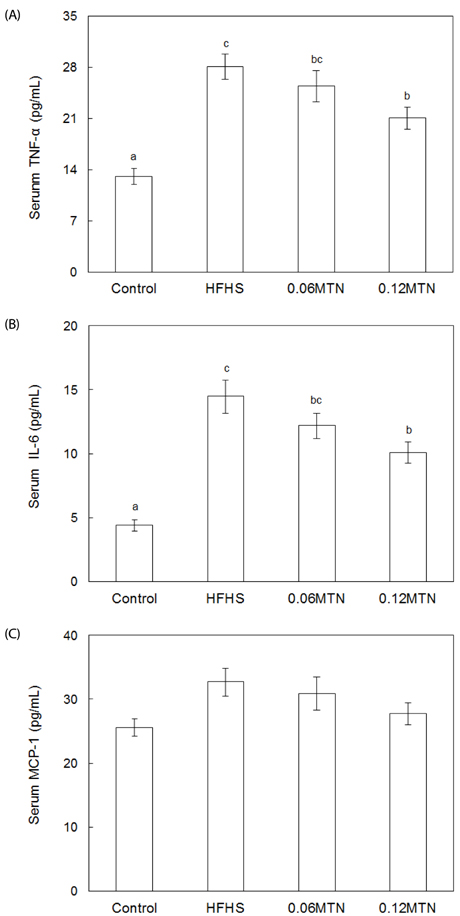
Myricetin given at 0.12% of the total diet significantly reduced body weight, weight gain, and epidydimal white adipose tissue weight, and improved hypertriglyceridemia and hypercholesterolemia without a significant influence on food intake in mice fed the HFHS diet. Serum glucose and insulin levels, as well as HOMA-IR values, decreased significantly by 0.12% myricetin supplementation in mice fed the HFHS diet. Myricetin given at 0.12% of the total diet significantly reduced serum levels of leptin, tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) in mice fed the HFHS diet.
CONCLUSIONS
These findings suggest that myricetin may have a protective effect against diet-induced obesity and insulin resistance in mice fed HFHS diet, and that alleviation of insulin resistance could partly occur by improving obesity and reducing serum proinflammatory cytokine levels.
Keyword
MeSH Terms
-
Adipose Tissue, White
Adiposity
Animals
Blood Glucose
Body Weight
Cytokines
Diabetes Mellitus, Type 2
Diet*
Eating
Glucose
Homeostasis
Humans
Hypercholesterolemia
Hypertriglyceridemia
Inflammation
Insulin
Insulin Resistance*
Interleukin-6
Leptin
Male
Mice*
Obesity
Risk Factors
Tumor Necrosis Factor-alpha
Weight Gain
Cytokines
Glucose
Insulin
Interleukin-6
Leptin
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Hypoglycemic and antioxidant effects of Daraesoon (Actinidia arguta shoot) in animal models of diabetes mellitus
Ah-Yeon Lee, Min-Jung Kang, Eunok Choe, Jung-In Kim
Nutr Res Pract. 2015;9(3):262-267. doi: 10.4162/nrp.2015.9.3.262.
Reference
-
1. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011; 378:31–40.
Article2. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999; 104:787–794.
Article3. Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. 2001; 109:Suppl 2. S135–S148.
Article4. Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001; 24:683–689.
Article5. Garg A, Grundy SM. Management of dyslipidemia in NIDDM. Diabetes Care. 1990; 13:153–169.
Article6. Lebovitz HE. Treating hyperglycemia in type 2 diabetes: new goals and strategies. Cleve Clin J Med. 2002; 69:809–820.
Article7. Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007; 21:1443–1455.
Article8. Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008; 94:206–218.
Article9. Ostlund RE Jr, Yang JW, Klein S, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab. 1996; 81:3909–3913.
Article10. Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994; 43:1271–1278.
Article11. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001; 280:E745–E751.
Article12. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001; 86:1930–1935.
Article13. Sheng T, Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genomics. 2008; 35:321–326.
Article14. Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008; 19:371–379.
Article15. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003; 112:1821–1830.
Article16. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006; 116:1494–1505.
Article17. Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 2006; 54:9966–9977.
Article18. Lin J, Zhang SM, Wu K, Willett WC, Fuchs CS, Giovannucci E. Flavonoid intake and colorectal cancer risk in men and women. Am J Epidemiol. 2006; 164:644–651.
Article19. Ko SY. Myricetin suppresses LPS-induced MMP expression in human gingival fibroblasts and inhibits osteoclastogenesis by downregulating NFATc1 in RANKL-induced RAW 264.7 cells. Arch Oral Biol. 2012; 57:1623–1632.
Article20. Kang BY, Kim SH, Cho D, Kim TS. Inhibition of interleukin-12 production in mouse macrophages via decreased nuclear factor-kappaB DNA binding activity by myricetin, a naturally occurring flavonoid. Arch Pharm Res. 2005; 28:274–279.
Article21. Lee YS, Choi EM. Myricetin inhibits IL-1beta-induced inflammatory mediators in SW982 human synovial sarcoma cells. Int Immunopharmacol. 2010; 10:812–814.
Article22. Liu IM, Tzeng TF, Liou SS, Lan TW. Myricetin, a naturally occurring flavonol, ameliorates insulin resistance induced by a high-fructose diet in rats. Life Sci. 2007; 81:1479–1488.
Article23. Chang CJ, Tzeng TF, Liou SS, Chang YS, Liu IM. Myricetin increases hepatic peroxisome proliferator-activated receptor α protein expression and decreases plasma lipids and adiposity in rats. Evid Based Complement Alternat Med. 2012; 2012:787152.24. Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988; 37:1163–1167.
Article25. Surwit RS, Seldin MF, Kuhn CM, Cochrane C, Feinglos MN. Control of expression of insulin resistance and hyperglycemia by different genetic factors in diabetic C57BL/6J mice. Diabetes. 1991; 40:82–87.
Article26. Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obesity and diabetes in mice. Am J Physiol Endocrinol Metab. 2002; 282:E207–E214.27. Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997; 20:1087–1092.
Article28. Yang ZH, Miyahara H, Takeo J, Katayama M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol Metab Syndr. 2012; 4:32.
Article29. Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr. 2003; 22:331–339.
Article30. Sato A, Kawano H, Notsu T, Ohta M, Nakakuki M, Mizuguchi K, Itoh M, Suganami T, Ogawa Y. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes. 2010; 59:2495–2504.
Article31. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995; 269:540–543.
Article32. Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010; 21:643–651.
Article33. Unger RH. Lipotoxic diseases. Annu Rev Med. 2002; 53:319–336.
Article34. Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem. 2003; 278:13740–13746.
Article35. Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008; 29:2959–2971.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mechanism of Insulin Resistance : Time Dependence of the Development of Insulin Resistance in High Fat Fed Rats
- Differential Effects of High-carbohydrate and High-fat Diet Composition on Muscle Insulin Resistance in Rats
- Effects of autumn olive berry on insulin resistance and hyperglycemia in mice fed a high-fat, high-sucrose diet
- Insulin Resistance of Skeletal Muscle was Recovered by Leptin Injection in vivo, but not in vitro, in High-fat Diet Fed Rats
- Effects of d-alpha-tocopherol supplements on lipid metabolism in a high-fat diet-fed animal model