Nutr Res Pract.
2014 Aug;8(4):404-409.
Effect of Hominis Placenta on cutaneous wound healing in normal and diabetic mice
- Affiliations
-
- 1Studies of Translational Acupuncture Research, Acupuncture and Meridian Science Research Center, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 130-701, Korea. acufind@khu.ac.kr
- 2Department of Korean Medical Science, Graduate School of Korean Medicine, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 130-701, Korea.
- 3Happy Kyung Hee Korean Medicine Clinic, Banrim-dong, Sungsan-gu, Changwon, Kyungbuk 642-180, Korea.
- 4Department of Food and Nutrition, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 130-701, Korea.
Abstract
- BACKGROUND/OBJECTIVES
The number of diabetic patients has recently shown a rapid increase, and delayed wound healing is a major clinical complication in diabetes. In this study, the wound healing effect of Hominis placenta (HP) treatment was investigated in normal and streptozotocin-induced diabetic mice.
MATERIALS/METHODS
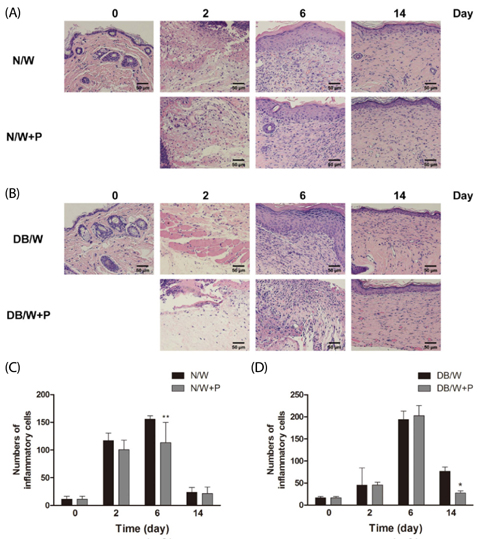
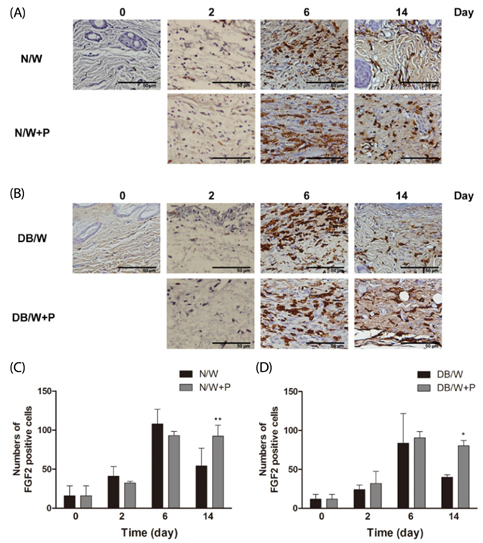
Four full thickness wounds were created using a 4 mm biopsy punch on the dorsum. HP was injected subcutaneously at the middle region of the upper and lower wounds. Wounds were digitally photographed and wound size was measured every other day until the 14th day. Wound closure rate was analyzed using CANVAS 7SE software. Wound tissues were collected on days 2, 6, and 14 after wounding for H/E, immunohistochemistry for FGF2, and Masson's trichrome staining for collagen study.
RESULTS
Significantly faster wound closure rates were observed in the HP treated group than in normal and diabetes control mice on days 6 and 8. Treatment with HP resulted in reduced localization of inflammatory cells in wounded skin at day 6 in normal mice and at day 14 in diabetic mice (P < 0.01). Expression of fibroblast growth factor (FGF) 2 showed a significant increase in the HP treated group on day 14 in both normal (P < 0.01) and diabetic mice (P < 0.05). In addition, HP treated groups showed a thicker collagen layer than no treatment groups, which was remarkable on the last day, day 14, in both normal and diabetic mice.
CONCLUSIONS
Taken together, HP treatment has a beneficial effect on acceleration of cutaneous wound healing via regulation of the entire wound healing process, including inflammation, proliferation, and remodeling.
MeSH Terms
Figure
Reference
-
1. Morain WD, Colen LB. Wound healing in diabetes mellitus. Clin Plast Surg. 1990; 17:493–501.
Article2. Hehenberger K, Heilborn JD, Brismar K, Hansson A. Inhibited proliferation of fibroblasts derived from chronic diabetic wounds and normal dermal fibroblasts treated with high glucose is associated with increased formation of l-lactate. Wound Repair Regen. 1998; 6:135–141.
Article3. McLaughlin PJ, Immonen JA, Zagon IS. Topical naltrexone accelerates full-thickness wound closure in type 1 diabetic rats by stimulating angiogenesis. Exp Biol Med (Maywood). 2013; 238:733–743.
Article4. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008; 453:314–321.
Article5. Witte MB, Barbul A. Role of nitric oxide in wound repair. Am J Surg. 2002; 183:406–412.
Article6. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004; 9:283–289.
Article7. Steed DL, Donohoe D, Webster MW, Lindsley L. Diabetic Ulcer Study Group. Effect of extensive debridement and treatment on the healing of diabetic foot ulcers. J Am Coll Surg. 1996; 183:61–64.8. Firat ET, Dag A, Gunay A, Kaya B, Karadede MI, Kanay BE, Ketani A, Evliyaoglu O, Uysal E. The effects of low-level laser therapy on palatal mucoperiosteal wound healing and oxidative stress status in experimental diabetic rats. Photomed Laser Surg. 2013; 31:315–321.
Article9. Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005; 67:3–21.
Article10. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003; 60:107–114.
Article11. Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes J, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987; 325:257–259.
Article12. Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986; 83:7297–7301.
Article13. Goldfarb G, Doan Ba T, Duran A. Human placenta for chronic leg ulcers. Lancet. 1980; 2:40.
Article14. Jang SY, Park JW, Bu Y, Kang JO, Kim J. Protective effects of hominis placenta hydrolysates on radiation enteropathy in mice. Nat Prod Res. 2011; 25:1988–1992.
Article15. Park SY, Phark S, Lee M, Lim JY, Sul D. Anti-oxidative and anti-inflammatory activities of placental extracts in benzo[a]pyrene-exposed rats. Placenta. 2010; 31:873–879.
Article16. De D, Chakraborty PD, Bhattacharyya D. Regulation of trypsin activity by peptide fraction of an aqueous extract of human placenta used as wound healer. J Cell Physiol. 2011; 226:2033–2040.
Article17. De D, Datta Chakraborty P, Mitra J, Sharma K, Mandal S, Das A, Chakrabarti S, Bhattacharyya D. Ubiquitin-like protein from human placental extract exhibits collagenase activity. PLoS One. 2013; 8:e59585.
Article18. Hong JW, Lee WJ, Hahn SB, Kim BJ, Lew DH. The effect of human placenta extract in a wound healing model. Ann Plast Surg. 2010; 65:96–100.
Article19. Shen FY, Lee MS, Jung SK. Effectiveness of pharmacopuncture for asthma: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2011; 2011.
Article20. Yeom MJ, Lee HC, Kim GH, Shim I, Lee HJ, Hahm DH. Therapeutic effects of Hominis placenta injection into an acupuncture point on the inflammatory responses in subchondral bone region of adjuvant-induced polyarthritic rat. Biol Pharm Bull. 2003; 26:1472–1477.
Article21. Seo TB, Han IS, Yoon JH, Seol IC, Kim YS, Jo HK, An JJ, Hong KE, Seo YB, Kim DH, Park SK, Yang DC, Namgung U. Growth-promoting activity of Hominis Placenta extract on regenerating sciatic nerve. Acta Pharmacol Sin. 2006; 27:50–58.
Article22. Lee KW, Ji HM, Kim DW, Choi SM, Kim S, Yang EJ. Effects of Hominis placenta on LPS-induced cell toxicity in BV2 microglial cells. J Ethnopharmacol. 2013; 147:286–292.
Article23. Peplow PV, Baxter GD. Gene expression and release of growth factors during delayed wound healing: a review of studies in diabetic animals and possible combined laser phototherapy and growth factor treatment to enhance healing. Photomed Laser Surg. 2012; 30:617–636.
Article24. Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest. 2010; 120:4207–4219.
Article25. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005; 366:1736–1743.
Article26. Tsourdi E, Barthel A, Rietzsch H, Reichel A, Bornstein SR. Current aspects in the pathophysiology and treatment of chronic wounds in diabetes mellitus. Biomed Res Int. 2013; 2013:385641.
Article27. Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007; 170:1178–1191.
Article28. Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002; 108:122–128.
Article29. Peppa M, Stavroulakis P, Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009; 17:461–472.
Article30. Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005; 31:674–686.
Article31. Roberts AB. Transforming growth factor-beta: activity and efficacy in animal models of wound healing. Wound Repair Regen. 1995; 3:408–418.
Article32. Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990; 136:1235–1246.33. Matsuda H, Koyama H, Sato H, Sawada J, Itakura A, Tanaka A, Matsumoto M, Konno K, Ushio H, Matsuda K. Role of nerve growth factor in cutaneous wound healing: accelerating effects in normal and healing-impaired diabetic mice. J Exp Med. 1998; 187:297–306.
Article34. Yoshida S, Matsumoto K, Tomioka D, Bessho K, Itami S, Yoshikawa K, Nakamura T. Recombinant hepatocyte growth factor accelerates cutaneous wound healing in a diabetic mouse model. Growth Factors. 2004; 22:111–119.
Article35. Eppley BL, Doucet M, Connolly D, Feder J. Enhancement of angiogenesis by bFGF in mandibular bone graft healing in the rabbit. J Oral Maxillofac Surg. 1988; 46:391–398.
Article36. Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg. 2003; 30:37–45.
Article37. Spanheimer RG, Umpierrez GE, Stumpf V. Decreased collagen production in diabetic rats. Diabetes. 1988; 37:371–376.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Human Adipose-derived Stem Cells on Cutaneous Wound Healing in Nude Mice
- Neuropeptide Y Promotes the Treatment of Adipose Stem Cells on Type 2 Diabetic Wounds
- Polysaccharides isolated from Phellinus gilvus enhances dermal wound healing in streptozotocin-induced diabetic rats
- Impaired Wound Healing in Diabetes Mellitus
- Reduced Expression of YAP in Dermal Fibroblasts is Associated with Impaired Wound Healing in Type 2 Diabetic Mice