Nutr Res Pract.
2014 Aug;8(4):386-390.
Protective effects of Acanthopanax divaricatus extract in mouse models of Alzheimer's disease
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Hallym University, 1 Hallymdaehak-gil, Chuncheon, Gangwon 200-702, Korea. dksong@hallym.ac.kr
Abstract
- BACKGROUND
Acanthopanax divaricatus var. albeofructus (ADA) extract has been reported to have anti-oxidant, immunomodulatory, and anti-mutagenic activity.
MATERIALS/METHODS
We investigated the effects of ADA extract on two mouse models of Alzheimer's disease (AD); intracerebroventricular injection of beta-amyloid peptide (Abeta) and amyloid precursor protein/presenilin 1 (APP/PS1)-transgenic mice.
RESULTS
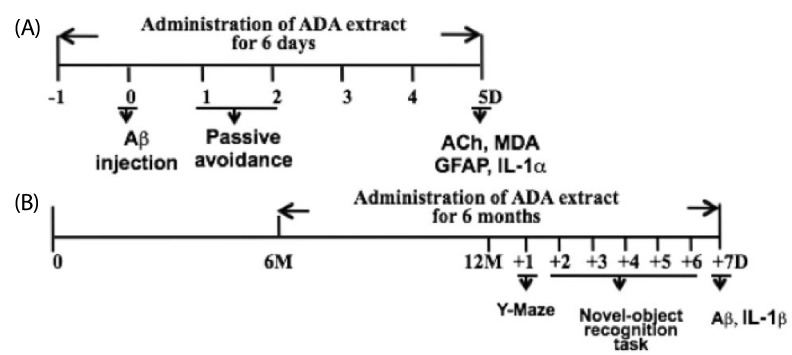
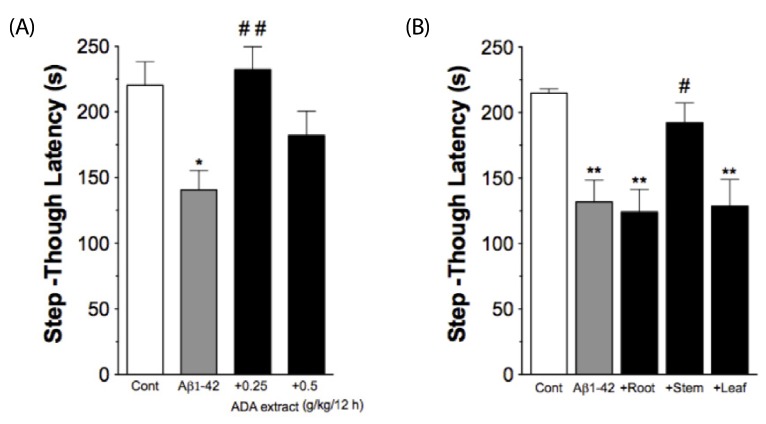
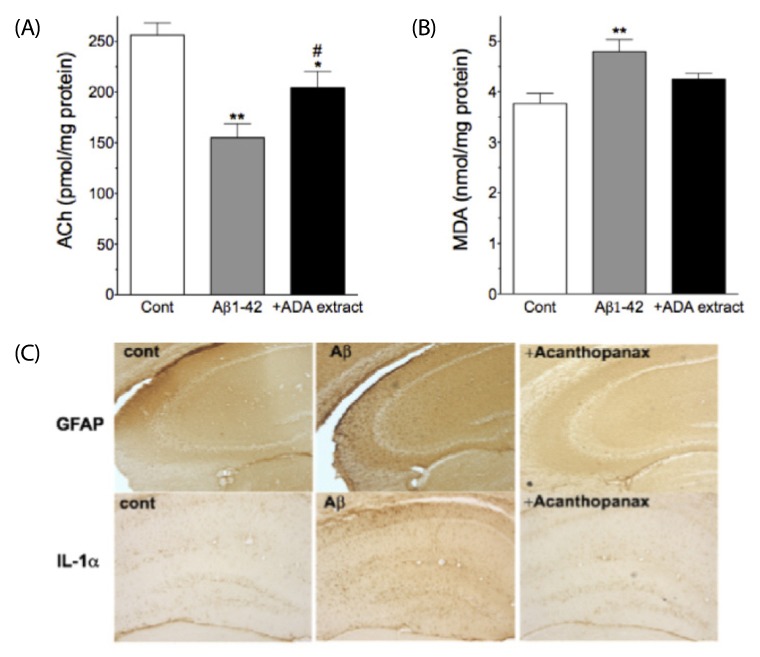
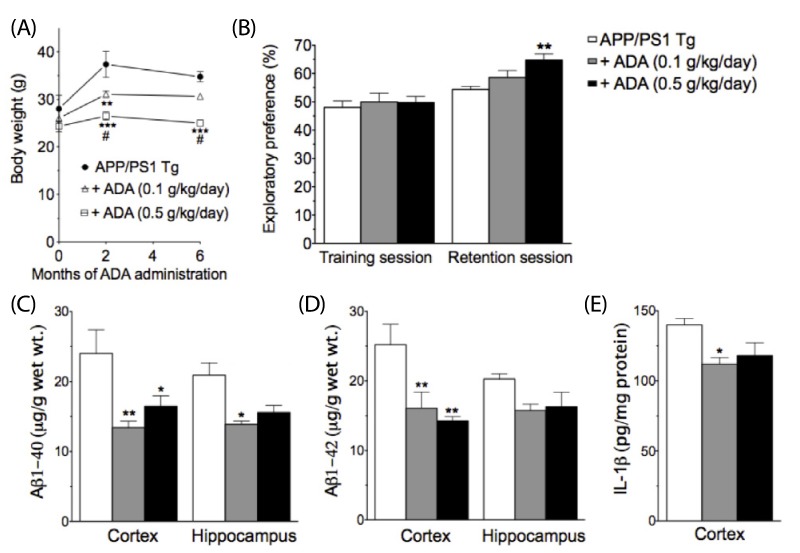
Intra-gastric administration of ADA stem extract (0.25 g/kg, every 12 hrs started from one day prior to injection of Abeta1-42 until evaluation) effectively blocked Abeta1-42-induced impairment in passive avoidance performance, and Abeta1-42-induced increase in immunoreactivities of glial fibrillary acidic protein and interleukin (IL)-1alpha in the hippocampus. In addition, it alleviated the Abeta1-42-induced decrease in acetylcholine and increase in malondialdehyde levels in the cortex. In APP/PS1-transgenic mice, chronic oral administration of ADA stem extract (0.1 or 0.5 g/kg/day for six months from the age of six to 12 months) resulted in significantly enhanced performance of the novel-object recognition task, and reduced amyloid deposition and IL-1beta in the brain.
CONCLUSIONS
The results of this study suggest that ADA stem extract may be useful for prevention and treatment of AD.
Keyword
MeSH Terms
Figure
Reference
-
1. Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012; 148:1204–1222. PMID: 22424230.
Article2. Maltsev AV, Bystryak S, Galzitskaya OV. The role of β-amyloid peptide in neurodegenerative diseases. Ageing Res Rev. 2011; 10:440–452. PMID: 21406255.
Article3. Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010; 6:108–119. PMID: 20140000.4. Yan JJ, Cho JY, Kim HS, Kim KL, Jung JS, Huh SO, Suh HW, Kim YH, Song DK. Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br J Pharmacol. 2001; 133:89–96. PMID: 11325798.5. Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001; 21:8370–8377. PMID: 11606625.
Article6. Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, Coppola D, Morgan D, Gordon MN. Microglial activation and beta-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002; 22:2246–2254. PMID: 11896164.7. Brekhman II, Dardymov IV. New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969; 9:419–430. PMID: 4892434.
Article8. Yook CS. Medicinal Herbs of Acanthopanax in Asia. Seoul: Kyeongwon Media;2001.9. Kim JY, Yang KS. Screening of antioxidant activity of Acanthopanax species in vitro. Yakhak Hoeji. 2003; 47:361–364.10. Lyu SY, Park WB. Th1/Th2 cytokine modulation in human PBMC by Acanthopanax divaricatus var. albeofructus. Food Sci Biotechnol. 2008; 17:631–636.11. Hong CE, Cho MC, Jang HA, Lyu SY. Mutagenicity and antimutagenicity of Acanthopanax divaricatus var. albeofructus. J Toxicol Sci. 2011; 36:661–668. PMID: 22008541.
Article12. Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986; 16:355–357. PMID: 3784576.13. Sarter M, Bodewitz G, Stephens DN. Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology (Berl). 1988; 94:491–495. PMID: 2836875.14. Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999; 401:63–69. PMID: 10485705.
Article15. Israël M, Lesbats B. Application to mammalian tissues of the chemiluminescent method for detecting acetylcholine. J Neurochem. 1982; 39:248–250. PMID: 7045285.
Article16. Baker H, Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neuroscience. 1993; 52:115–134. PMID: 8094544.
Article17. Cho JY, Kim HS, Kim DH, Yan JJ, Suh HW, Song DK. Inhibitory effects of long-term administration of ferulic acid on astrocyte activation induced by intracerebroventricular injection of beta-amyloid peptide (1-42) in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2005; 29:901–907. PMID: 15970368.18. Fu AL, Dong ZH, Sun MJ. Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res. 2006; 1109:201–206. PMID: 16872586.19. Webber KM, Casadesus G, Perry G, Atwood CS, Bowen R, Smith MA. Gender differences in Alzheimer disease: the role of luteinizing hormone in disease pathogenesis. Alzheimer Dis Assoc Disord. 2005; 19:95–99. PMID: 15942328.20. Kim HS, Cho JY, Kim DH, Yan JJ, Lee HK, Suh HW, Song DK. Inhibitory effects of long-term administration of ferulic acid on microglial activation induced by intracerebroventricular injection of beta-amyloid peptide (1-42) in mice. Biol Pharm Bull. 2004; 27:120–121. PMID: 14709913.
Article21. Dumont M, Beal MF. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic Biol Med. 2011; 51:1014–1026. PMID: 21130159.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- First Report and Characterization of Pestalotiopsis ellipsospora Causing Canker on Acanthopanax divaricatus
- Yield Analysis of Flavonoids in Acanthopanax divaricatus and A. koreanum Grown using Different Cultivation Methods
- Inhibition of Lung Inflammation by Acanthopanax divaricatus var. Albeofructus and Its Constituents
- The Effect of Acanthopanax koreanum Extract on the Induction of Collagen Induced Arthritis for DBA/1J Mice
- Acanthopanax sessiliflorus stem confers increased resistance to environmental stresses and lifespan extension in Caenorhabditis elegans