Nutr Res Pract.
2014 Aug;8(4):352-359.
Anti-inflammatory effect of methanol extract from Erigeron Canadensis L. may be involved with upregulation of heme oxygenase-1 expression and suppression of NFkappaB and MAPKs activation in macrophages
- Affiliations
-
- 1Department of Food Science and Biotechnology, Chungbuk National University, 52 Naesudong-ro, Heungdeok-gu, Cheongju, Chungbuk 361-763, Korea. junsoo@chungbuk.ac.kr
Abstract
- BACKGROUND/OBJECTIVES
In this study, we determined the anti-inflammatory activities and the underlying molecular mechanisms of the methanol extract from Erigeron Canadensis L. (ECM) in LPS-stimulated RAW264.7 macrophage cells.
MATERIALS/METHODS
The potential anti-inflammatory properties of ECM were investigated by using RAW264.7 macrophages. We used western blot assays and real time quantitative polymerase chain reaction to detect protein and mRNA expression, respectively. Luciferase assays were performed to determine the transactivity of transcription factors.
RESULTS
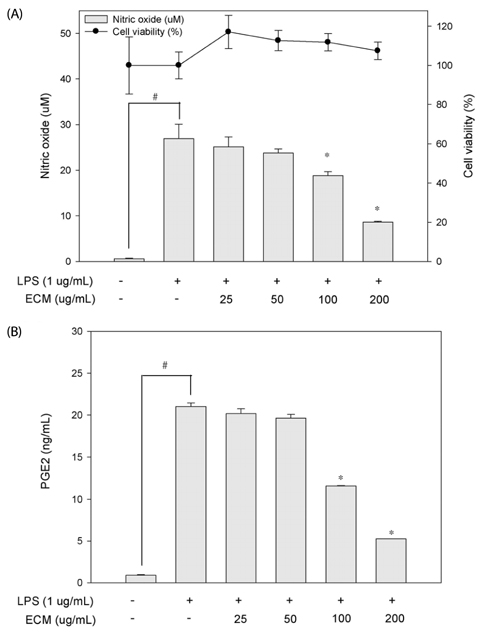
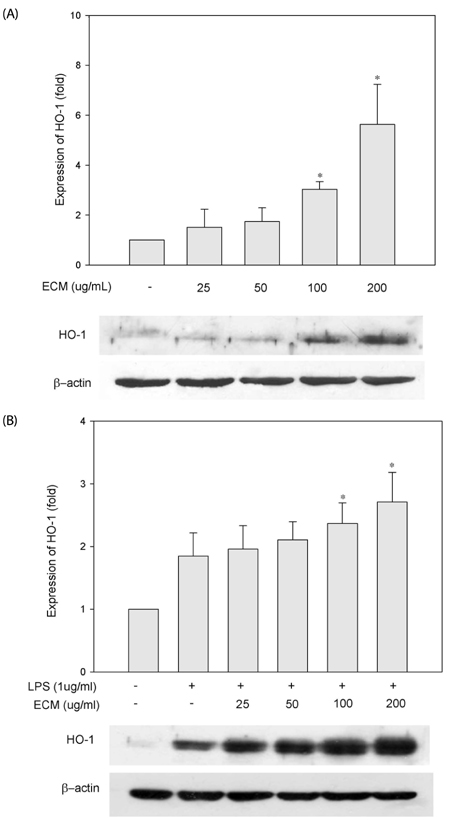
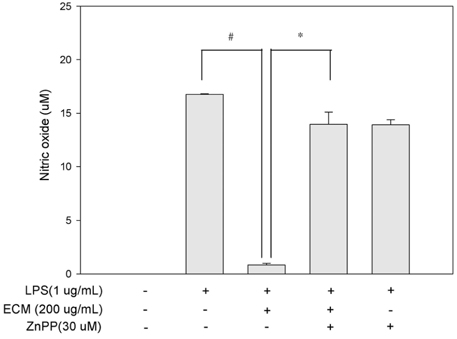
ECM significantly inhibited inducible nitric oxide synthase (iNOS)-derived NO and cyclooxygenase-2 (COX-2) derived PGE2 production in LPS-stimulated RAW264.7 macrophages. These inhibitory effects of ECM were accompanied by decreases in LPS-induced nuclear translocations and transactivities of NFkappaB. Moreover, phosphorylation of mitogen-activated protein kinase (MAPKs) including extracellular signal-related kinase (ERK1/2), p38, and c-jun N-terminal kinase (JNK) was significantly suppressed by ECM in LPS-stimulated RAW264.7 macrophages. Further studies demonstrated that ECM by itself induced heme oxygenase-1 (HO-1) protein expression at the protein levels in dose-dependent manner. However, zinc protoporphyrin (ZnPP), a selective HO-1 inhibitor, abolished the ECM-induced suppression of NO production.
CONCLUSIONS
These results suggested that ECM-induced HO-1 expression was partly responsible for the resulting anti-inflammatory effects. These findings suggest that ECM exerts anti-inflammatory actions and help to elucidate the mechanisms underlying the potential therapeutic values of Erigeron Canadensis L.
MeSH Terms
-
Blotting, Western
Cyclooxygenase 2
Dinoprostone
Erigeron*
Heme Oxygenase-1*
JNK Mitogen-Activated Protein Kinases
Luciferases
Macrophages*
Methanol*
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphorylation
Phosphotransferases
Polymerase Chain Reaction
Protein Kinases
RNA, Messenger
Transcription Factors
Up-Regulation*
Zinc
Cyclooxygenase 2
Dinoprostone
Heme Oxygenase-1
JNK Mitogen-Activated Protein Kinases
Luciferases
Methanol
Nitric Oxide
Nitric Oxide Synthase Type II
Phosphotransferases
Protein Kinases
RNA, Messenger
Transcription Factors
Zinc
Figure
Reference
-
1. Joh EH, Gu W, Kim DH. Echinocystic acid ameliorates lung inflammation in mice and alveolar macrophages by inhibiting the binding of LPS to TLR4 in NF-κB and MAPK pathways. Biochem Pharmacol. 2012; 84:331–340.
Article2. Lee JA, Lee MY, Shin IS, Seo CS, Ha H, Shin HK. Anti-inflammatory effects of Amomum compactum on RAW 264.7 cells via induction of heme oxygenase-1. Arch Pharm Res. 2012; 35:739–746.
Article3. Kim SH, Shin TY. Anti-inflammatory effect of leaves of Eriobotrya japonica correlating with attenuation of p38 MAPK, ERK, and NF-kappaB activation in mast cells. Toxicol In Vitro. 2009; 23:1215–1219.
Article4. Ku KT, Huang YL, Huang YJ, Chiou WF. Miyabenol A inhibits LPS-induced NO production via IKK/IkappaB inactivation in RAW 264.7 macrophages: possible involvement of the p38 and PI3K pathways. J Agric Food Chem. 2008; 56:8911–8918.
Article5. Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001; 22:153–183.
Article6. Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990; 10:1498–1506.
Article7. Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son JK, Shin HK. Anti-inflammatory activity of Angelica dahurica ethanolic extract on RAW264.7 cells via upregulation of heme oxygenase-1. Food Chem Toxicol. 2011; 49:1047–1055.
Article8. Chen TY, Sun HL, Yao HT, Lii CK, Chen HW, Chen PY, Li CC, Liu KL. Suppressive effects of Indigofera suffruticosa Mill extracts on lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 macrophages. Food Chem Toxicol. 2013; 55:257–264.
Article9. Kim Y, Sung J, Sung M, Choi Y, Jeong HS, Lee J. Involvement of heme oxygenase-1 in the anti-inflammatory activity of Chrysanthemum boreale Makino extracts on the expression of inducible nitric oxide synthase in RAW264.7 macrophages. J Ethnopharmacol. 2010; 131:550–554.
Article10. Pawlaczyk I, Czerchawski L, Kuliczkowski W, Karolko B, Pilecki W, Witkiewicz W, Gancarz R. Anticoagulant and anti-platelet activity of polyphenolic-polysaccharide preparation isolated from the medicinal plant Erigeron canadensis L. Thromb Res. 2011; 127:328–340.
Article11. Mukhtar N, Iqbal K, Malik A. Novel sphingolipids from Conyza canadensis. Chem Pharm Bull (Tokyo). 2002; 50:1558–1560.
Article12. Veres K, Csupor-Löffler B, Lázár A, Hohmann J. Antifungal activity and composition of essential oils of Conyza canadensis herbs and roots. ScientificWorldJournal. 2012; 2012:489646.13. Xie WD, Gao X, Jia ZJ. A new C-10 acetylene and a new triterpenoid from Conyza canadensis. Arch Pharm Res. 2007; 30:547–551.
Article14. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986; 89:271–277.15. Jung CH, Kim JH, Hong MH, Seog HM, Oh SH, Lee PJ, Kim GJ, Kim HM, Um JY, Ko SG. Phenolic-rich fraction from Rhus verniciflua Stokes (RVS) suppress inflammatory response via NF-kappaB and JNK pathway in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol. 2007; 110:490–497.
Article16. Chiu FL, Lin JK. Tomatidine inhibits iNOS and COX-2 through suppression of NF-kappaB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008; 582:2407–2412.
Article17. Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, Song JC, Jeong KS. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem. 2003; 243:153–160.18. Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J Biol Chem. 1999; 274:30858–30863.
Article19. Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002; 234-235:249–263.
Article20. Kim AN, Jeon WK, Lee JJ, Kim BC. Up-regulation of heme oxygenase-1 expression through CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic Biol Med. 2010; 49:323–331.
Article21. Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater. 2006; 1:R1–R9.
Article22. Li B, Lee DS, Choi HG, Kim KS, Kang DG, Lee HS, Jeong GS, Kim YC. Sauchinone suppresses pro-inflammatory mediators by inducing heme oxygenase-1 in RAW264.7 macrophages. Biol Pharm Bull. 2011; 34:1566–1571.
Article23. Wang Z, Jiang W, Zhang Z, Qian M, Du B. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J Ethnopharmacol. 2012; 144:145–150.
Article24. Cheon MS, Yoon T, Lee do Y, Choi G, Moon BC, Lee AY, Choo BK, Kim HK. Chrysanthemum indicum Linné extract inhibits the inflammatory response by suppressing NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol. 2009; 122:473–477.
Article25. Paul A, Cuenda A, Bryant CE, Murray J, Chilvers ER, Cohen P, Gould GW, Plevin R. Involvement of mitogen-activated protein kinase homologues in the regulation of lipopolysaccharide-mediated induction of cyclo-oxygenase-2 but not nitric oxide synthase in RAW 264.7 macrophages. Cell Signal. 1999; 11:491–497.
Article26. Choi HG, Lee DS, Li B, Choi YH, Lee SH, Kim YC. Santamarin, a sesquiterpene lactone isolated from Saussurea lappa, represses LPS-induced inflammatory responses via expression of heme oxygenase-1 in murine macrophage cells. Int Immunopharmacol. 2012; 13:271–279.
Article27. Bang SY, Kim JH, Kim HY, Lee YJ, Park SY, Lee SJ, Kim Y. Achyranthes japonica exhibits anti-inflammatory effect via NF-κB suppression and HO-1 induction in macrophages. J Ethnopharmacol. 2012; 144:109–117.
Article28. Jin GH, Park SY, Kim E, Ryu EY, Kim YH, Park G, Lee SJ. Anti-inflammatory activity of Bambusae Caulis in Taeniam through heme oxygenase-1 expression via Nrf-2 and p38 MAPK signaling in macrophages. Environ Toxicol Pharmacol. 2012; 34:315–323.
Article29. Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Can'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999; 274:26071–26078.
Article30. Hwangbo C, Lee HS, Park J, Choe J, Lee JH. The anti-inflammatory effect of tussilagone, from Tussilago farfara, is mediated by the induction of heme oxygenase-1 in murine macrophages. Int Immunopharmacol. 2009; 9:1578–1584.
Article31. Srisook K, Palachot M, Mongkol N, Srisook E, Sarapusit S. Anti-inflammatory effect of ethyl acetate extract from Cissus quadrangularis Linn may be involved with induction of heme oxygenase-1 and suppression of NF-kappaB activation. J Ethnopharmacol. 2011; 133:1008–1014.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- The Stem Bark of Kalopanax pictus Exhibits Anti-inflammatory Effect through Heme Oxygenase-1 Induction and NF-kappaB Suppression
- Suppression of Heme Oxygenase-1 by Prostaglandin E2-Protein Kinase A-A-Kinase Anchoring Protein Signaling Is Central for Augmented Cyclooxygenase-2 Expression in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages
- Antioxidant and anti-inflammatory effects and mechanism of Abeliophyllum distichum leaf extract in RAW264.7 macrophages
- The Anti-Inflammatory Activity of Eucommia ulmoides Oliv. Bark. Involves NF-κB Suppression and Nrf2-Dependent HO-1 Induction in BV-2 Microglial Cells