Nutr Res Pract.
2014 Feb;8(1):40-45.
Hepatoprotective effects of Rubus coreanus miquel concentrates on liver injuries induced by carbon tetrachloride in rats
- Affiliations
-
- 1Department of Food and Nutrition, Inha University, 100 Inha-ro, Namgu, Inchon 402-751, Korea. jhchyun@inha.ac.kr
- 2Department of Food and Nutrition, Changwon National University, Gyeongnam 641-773, Korea.
- 3Department of Pharmacy, Kyung Hee University, Seoul 130-701, Korea.
Abstract
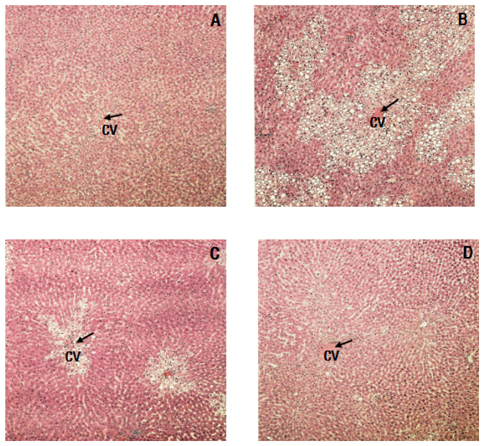
- As well-being foods pursuing healthy life are becoming popular, interest in Rubus coreanus Miquel (RCM) fruit, a type of Korean blackberry, is increasing due to its medicinal actions including protecting the liver, brightening the eyes, and alleviating diabetes. This study was carried out to evaluate the hepatoprotective effects of RCM concentrates on liver injuries induced by carbon tetrachloride (CCl4) in rats. RCM, produced in June ~ July 2008 at Chunbook, Gochang (South Korea), was finely mashed. The seeds were removed and the juices were condensed. Thirty-two Sprague-Dawley rats were divided into four groups according to treatment: normal (eight rats), CCl4, 1% RCM, and 2% RCM. Experimental diets were provided to the experimental animals for 4 weeks. We measure total cholesterol, high density lipoprotein-cholesterol (HDL-C), aspartate amino transferase (AST), alanine amino transferase (ALT), and alkaline phosphatase (ALP) levels. Part of the livers was isolated for histopathological evaluation, and analyzed for lipid peroxide (TBARS), superoxide dismutase (SOD) and liver proteins. The activities of serum AST, ALT, and ALP were elevated following CCl4 administration. Levels of hepatic TBARS were also significantly increased in the CCl4 groups. However, hepatic TBARS levels and the activities of serum enzymes were markedly reduced by supplementation with the RCM concentrates (P < 0.05). Hepatic SOD activity increased in the RCM concentrates group versus CCl4 groups. Histopathological examination revealed massive necrosis in the centrilobular area and degenerative changes caused by CCl4 were ameliorated by dietary supplementation with RCM concentrates. These results suggest that RCM concentrates have hepatoprotective effects and may improve the symptoms of liver injuries.
MeSH Terms
-
Alanine
Alkaline Phosphatase
Animals
Aspartic Acid
Carbon Tetrachloride*
Carbon*
Cholesterol
Diet
Dietary Supplements
Fruit
Liver*
Necrosis
Rats*
Rats, Sprague-Dawley
Superoxide Dismutase
Thiobarbituric Acid Reactive Substances
Transferases
Alanine
Alkaline Phosphatase
Aspartic Acid
Carbon
Carbon Tetrachloride
Cholesterol
Superoxide Dismutase
Thiobarbituric Acid Reactive Substances
Transferases
Figure
Reference
-
1. Jun DW, Cho YK, Sohn JH, Lee CH, Kim SH, Eun JR. A study of the awareness of chronic liver diseases among Korean adults. Korean J Hepatol. 2011; 17:99–105.
Article2. Campos R, Garrido A, Guerra R, Valenzuela A. Silybin dihemisuccinate protects against glutathione depletion and lipid peroxidation induced by acetaminophen on rat liver. Planta Med. 1989; 55:417–419.
Article3. Ip SP, Yiu HY, Ko KM. Differential effect of schisandrin B and dimethyl diphenyl bicarboxylate (DDB) on hepatic mitochondrial glutathione redox status in carbon tetrachloride intoxicated mice. Mol Cell Biochem. 2000; 205:111–114.4. Kwak CS, Kim MY, Lee MS. Antioxidative effect of plant food mixtures in rat fed on high fat-high cholesterol diet. Korean J Nutr. 2005; 38:352–363.5. Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, Heber D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem. 2008; 56:1415–1422.
Article6. Fazio A, Plastina P, Meijerink J, Witkamp RF, Gabriele B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013; 140:817–824.
Article7. Jeon SK, Lee JW, Lee IS. Effect of antioxidant activity and induction of DNA damage of human gastric cancer cell by Rubus coreanus Miquel. J Life Sci. 2007; 17:1723–1728.
Article8. Lee JW, Do JH. Chemical compounds and volatile flavor of Rubus coreanum. Korean J Food Nutr. 2000; 13:453–459.9. Cha HS, Park MS, Park KM. Physiological activities of Rubus coreanus Miquel. Korean J Food Sci Technol. 2001; 33:409–415.10. Lee JE, Park E, Lee JE, Auh JH, Choi HK, Lee J, Cho S, Kim JH. Effects of a Rubus coreanus Miquel supplement on plasma antioxidant capacity in healthy Korean men. Nutr Res Pract. 2011; 5:429–434.
Article11. Lim JW, Hwang HJ, Shin CS. Polyphenol compounds and anti-inflammatory activities of Korean black raspberry (Rubus coreanus Miquel) wines produced from juice supplemented with pulp and seed. J Agric Food Chem. 2012; 60:5121–5127.
Article12. Ku CS, Mun SP. Antioxidant activities of ethanol extracts from seeds in fresh Bokbunja (Rubus coreanus Miq.) and wine processing waste. Bioresour Technol. 2008; 99:4503–4509.
Article13. Chou WH, Oinaka T, Kanamaru F, Mizutani K, Chen FH, Tanaka O. Diterpene glycosides from leaves of Chinese Rubus chingii and fruits of R. suavissimus, and identification of the source plant of the Chinese folk medicine "fu-pen-zi". Chem Pharm Bull (Tokyo). 1987; 35:3021–3024.
Article14. Hattori M, Kuo KP, Shu YZ, Tezuka Y, Kikuchi T, Namba T. A triterpene from the fruits of Rubus chingii. Phytochemistry. 1988; 27:3975–3976.
Article15. Ohtani K, Miyajima C, Takahasi T, Kasai R, Tanaka O, Hahn DR, Naruhashi N. A dimeric triterpene-glycoside fromRubus coreanus. Phytochemistry. 1990; 29:3275–3280.
Article16. Lim JD, Yu CY, Kim MJ, Yun SJ, Lee SJ, Kim NY, Chung IM. Comparision of SOD activity and phenolic compound contents in various Korean medicinal plants. Korean J Med Crop Sci. 2004; 12:191–202.17. Kwon KH, Cha WS, Kim DC, Shin HJ. A research and application of active ingredients in Bokbunja (Rubus coreanus Miquel). Korean J Biotechnol Bioeng. 2006; 21:405–409.18. Kim HS, Chyun JH. Effects of dietary protein level on catecholamine concentration of laparotomized rats. Korean J Nutr. 1992; 25:248–255.19. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957; 28:56–63.
Article20. Wada M, Nagano M, Kido H, Ikeda R, Kuroda N, Nakashima K. Suitability of TBA method for the evaluation of the oxidative effect of non-water-soluble and water-soluble rosemary extracts. J Oleo Sci. 2011; 60:579–584.
Article21. Peterson GL. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979; 100:201–220.
Article22. Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003; 189:113–127.
Article23. Sicilia T, Mally A, Schauer U, Pähler A, Völkel W. LC-MS/MS methods for the detection of isoprostanes (iPF2alpha-III and 8,12-iso-iPF2alpha-VI) as biomarkers of CCl4-induced oxidative damage to hepatic tissue. J Chromatogr B Analyt Technol Biomed Life Sci. 2008; 861:48–55.
Article24. Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003; 33:105–136.
Article25. Mourelle M, Meza MA. CCl4-induced lipoperoxidation triggers a lethal defect in the liver plasma membranes. J Appl Toxicol. 1990; 10:23–27.
Article26. Hsiao G, Shen MY, Lin KH, Lan MH, Wu LY, Chou DS, Lin CH, Su CH, Sheu JR. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J Agric Food Chem. 2003; 51:3302–3308.
Article27. Basarkar PW, Nath N. Cholesterol lowering action of vitamin P-like compounds in rats. Indian J Exp Biol. 1981; 19:787–789.28. Matsumoto N, Okushio K, Hara Y. Effect of black tea polyphenols on plasma lipids in cholesterol-fed rats. J Nutr Sci Vitaminol (Tokyo). 1998; 44:337–342.
Article29. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005; 172:367–379.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment for Two Cases of Acne Vulgaris with Rubus Coreanus Miquel Extract
- Growth inhibition effect of Rubus coreanus Miquel on Candida albicans
- Morphologic Change of Rat Liver Induced by Repeated Administration of Carbon Tetrachloride and Dimethylnitrosamine
- A Study of Potential Application of Rubus coreanus Miquel Extract for Seborrheic Dermatitis Treatment
- Carbon tetrachloride (CCl4)-induced hepatic fibrosis in the rat