Nutr Res Pract.
2013 Aug;7(4):302-308.
Glycemic index of dietary formula may not be predictive of postprandial endothelial inflammation: a double-blinded, randomized, crossover study in non-diabetic subjects
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Ewha Womans University, 52, Ewhayeodae-gil, Seodaemun-gu, Seoul 120-750, Korea. orank@ewha.ac.kr
- 2Department of Food Science and Technology, Seoul National University of Science and Technology, Seoul 139-743, Korea.
- 3Biofood Network, Seoul 120-160, Korea.
- 4Department of Family Medicine, Seoul St Mary's Hospital, Seoul 137-701, Korea.
Abstract
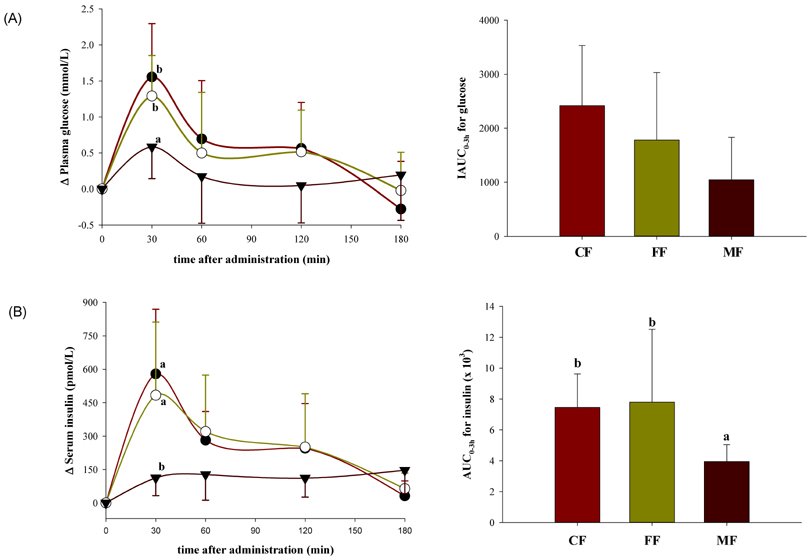
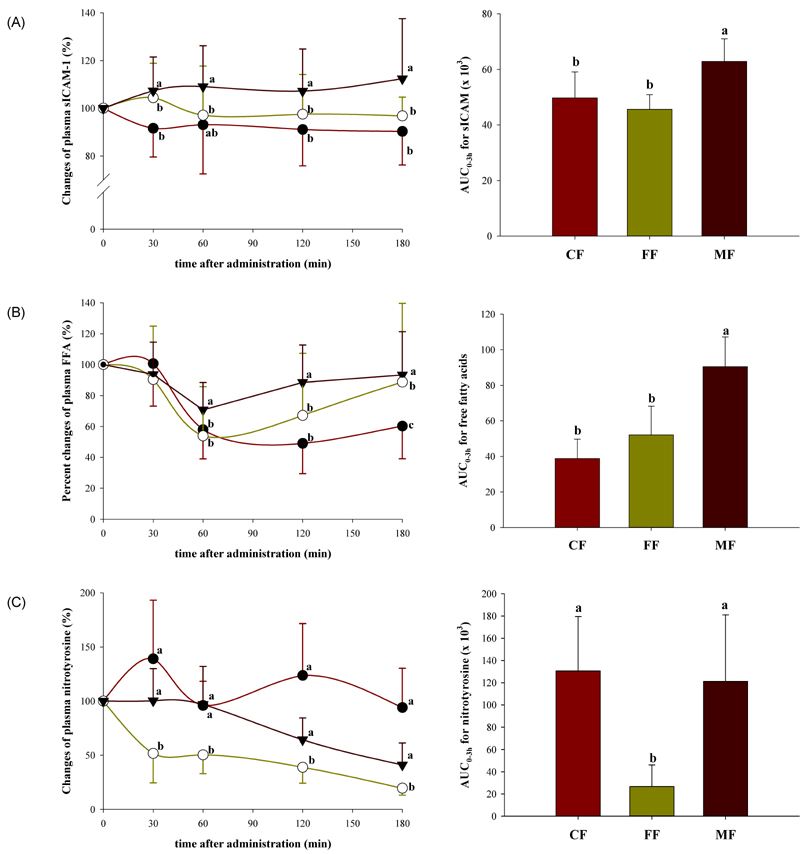
- The emerging role of endothelial inflammation in diabetes has stimulated research interest in the effects of nutrition on related indices. In the current study we investigated whether the nutrient composition of dietary formula as reflected in glycemic index (GI) may be predictive of postprandial endothelial inflammation in non-diabetic subjects. A double-blinded, randomized, crossover study was conducted in non-diabetic subjects (n = 8/group). Each subject consumed three types of diabetes-specific dietary formulas (high-fiber formula [FF], high-monounsaturated fatty acid (MUFA) formula [MF] and control formula [CF]) standardized to 50 g of available carbohydrates with a 1-week interval between each. The mean glycemic index (GI) was calculated and 3-hour postprandial responses of insulin, soluble intercellular adhesion molecule-1 (sICAM-1), nitrotyrosine (NT) and free fatty acids (FFA) were measured. The MF showed the lowest mean GI and significantly low area under the curve (AUC) for insulin (P = 0.038), but significantly high AUCs for sICAM-1 (P < 0.001) and FFA (P < 0.001) as compared to the CF and FF. The FF showed intermediate mean GI, but significantly low AUC for NT (P < 0.001) as compared to the CF and MF. The mean GI was not positively correlated to any of the inflammatory markers evaluated, and in fact negatively correlated to changes in FFA (r = -0.473, P = 0.006). While the MF with the lowest GI showed the highest values in most of the inflammatory markers measured, the FF with intermediate GI had a modest beneficial effect on endothelial inflammation. These results suggest that nutrient composition of dietary formula as reflected in the GI may differently influence acute postprandial inflammation in non-diabetic subjects.
MeSH Terms
Figure
Reference
-
1. Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981; 34:362–366.
Article2. Brand-Miller J, Wolever TM, Foster-Powell K, Colagiuri S. The New Glucose Revolution: The Authoritative Guide to the Glycemic Index--The Dietary Solution for Lifelong Health. New York (NY): Marlowe & Company;2003.3. Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008; 31:2281–2283.
Article4. World Health Organization. Food and Agriculture Organization of the United Nations. Carbohydrates in Human Nutrition. Report of a Joint FAO/WHO Expert Consultation. Rome: Food and Agriculture Organization of the United Nations;1998. p. 1–140.5. Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002; 76:274S–280S.
Article6. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005; 54:1–7.7. De Man FH, Cabezas MC, Van Barlingen HH, Erkelens DW, de Bruin TW. Triglyceride-rich lipoproteins in non-insulin-dependent diabetes mellitus: post-prandial metabolism and relation to premature atherosclerosis. Eur J Clin Invest. 1996; 26:89–108.
Article8. Rubin D, Claas S, Pfeuffer M, Nothnagel M, Foelsch UR, Schrezenmeir J. s-ICAM-1 and s-VCAM-1 in healthy men are strongly associated with traits of the metabolic syndrome, becoming evident in the postprandial response to a lipid-rich meal. Lipids Health Dis. 2008; 7:32.
Article9. Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011; 14:575–585.
Article10. Grundy SM, Howard B, Smith S Jr, Eckel R, Redberg R, Bonow RO. Prevention Conference VI: Diabetes and Cardiovascular Disease: executive summary: conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2002; 105:2231–2239.
Article11. Elia M, Ceriello A, Laube H, Sinclair AJ, Engfer M, Stratton RJ. Enteral nutritional support and use of diabetes-specific formulas for patients with diabetes: a systematic review and meta-analysis. Diabetes Care. 2005; 28:2267–2279.
Article12. Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002; 23:831–834.
Article13. Wolever TM, Jenkins DJ, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991; 54:846–854.
Article14. Lefèbvre PJ, Scheen AJ. The postprandial state and risk of cardiovascular disease. Diabet Med. 1998; 15:Suppl 4. S63–S68.
Article15. Yokoyama J, Someya Y, Yoshihara R, Ishii H. Effects of high-monounsaturated fatty acid enteral formula versus high-carbohydrate enteral formula on plasma glucose concentration and insulin secretion in healthy individuals and diabetic patients. J Int Med Res. 2008; 36:137–146.
Article16. Garg A. High-monounsaturated-fat diets for patients with diabetes mellitus: a meta-analysis. Am J Clin Nutr. 1998; 67:577S–582S.
Article17. Ceriello A. The post-prandial state and cardiovascular disease: relevance to diabetes mellitus. Diabetes Metab Res Rev. 2000; 16:125–132.
Article18. Ceriello A, Assaloni R, Da Ros R, Maier A, Piconi L, Quagliaro L, Esposito K, Giugliano D. Effect of atorvastatin and irbesartan, alone and in combination, on postprandial endothelial dysfunction, oxidative stress, and inflammation in type 2 diabetic patients. Circulation. 2005; 111:2518–2524.
Article19. Peairs AD, Rankin JW, Lee YW. Effects of acute ingestion of different fats on oxidative stress and inflammation in overweight and obese adults. Nutr J. 2011; 10:122.
Article20. Bevilacqua MP, Nelson RM, Mannori G, Cecconi O. Endothelial-leukocyte adhesion molecules in human disease. Annu Rev Med. 1994; 45:361–378.
Article21. Shishehbor MH, Aviles RJ, Brennan ML, Fu X, Goormastic M, Pearce GL, Gokce N, Keaney JF Jr, Penn MS, Sprecher DL, Vita JA, Hazen SL. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003; 289:1675–1680.
Article22. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000; 85:2970–2973.
Article23. Pilz S, März W. Free fatty acids as a cardiovascular risk factor. Clin Chem Lab Med. 2008; 46:429–434.
Article24. Onody A, Csonka C, Giricz Z, Ferdinandy P. Hyperlipidemia induced by a cholesterol-rich diet leads to enhanced peroxynitrite formation in rat hearts. Cardiovasc Res. 2003; 58:663–670.
Article25. Shimabukuro M, Chinen I, Higa N, Takasu N, Yamakawa K, Ueda S. Effects of dietary composition on postprandial endothelial function and adiponectin concentrations in healthy humans: a crossover controlled study. Am J Clin Nutr. 2007; 86:923–928.
Article26. Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002; 39:1145–1150.
Article27. Ceriello A, Quagliaro L, D'Amico M, Di Filippo C, Marfella R, Nappo F, Berrino L, Rossi F, Giugliano D. Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes. 2002; 51:1076–1082.
Article28. Lada AT, Rudel LL. Dietary monounsaturated versus polyunsaturated fatty acids: which is really better for protection from coronary heart disease? Curr Opin Lipidol. 2003; 14:41–46.
Article29. Chang NW, Huang PC. Effects of dietary monounsaturated fatty acids on plasma lipids in humans. J Lipid Res. 1990; 31:2141–2147.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glycemic Index Revisited
- Effect of Various Snacks and Meal woth Different Kinds of Staples on Bloos Glucose, Insulin, and C- Peptide Levels in Healthy and Type 2 Diabetic Patients
- Effects of Chili Treatment on Gastrointestinal and Rectal Sensation in Diarrhea-predominant Irritable Bowel Syndrome: A Randomized, Double-blinded, Crossover Study
- Effects of Sasa Borealis Leaf Extract on the Glucose Tolerance of Major Foods for Carbohydrate
- Effects of Low Glycemic Index Nutrition Education on the Blood Glucose Control in Patients with Type 2 Diabetes Mellitus