Nutr Res Pract.
2013 Aug;7(4):294-301.
Dietary carnosic acid suppresses hepatic steatosis formation via regulation of hepatic fatty acid metabolism in high-fat diet-fed mice
- Affiliations
-
- 1Functional Food and Nutrition Division, Department of Agrofood Resources, Rural Development Administration, Suwon 441-707, Korea.
- 2Department of Obstetrics and Gynecology, Soonchunhyang University Cheonan Hospital, 23-20, Byeongmyeong-dong, Dongnam-gu, Cheonan 330-721, Korea. sternun@schmc.ac.kr
Abstract
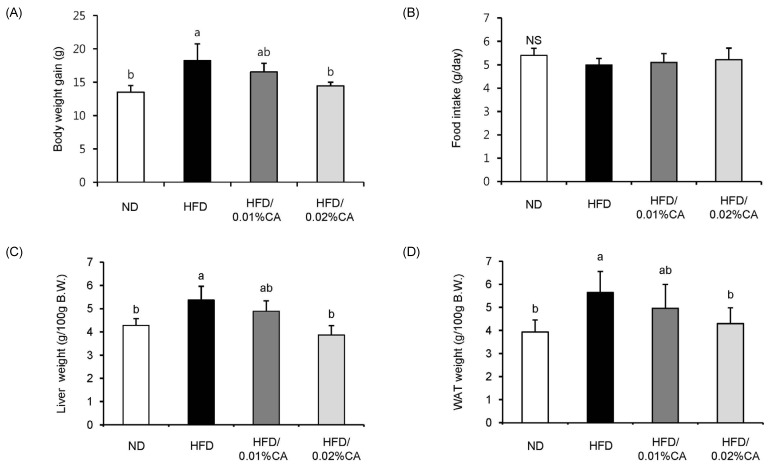
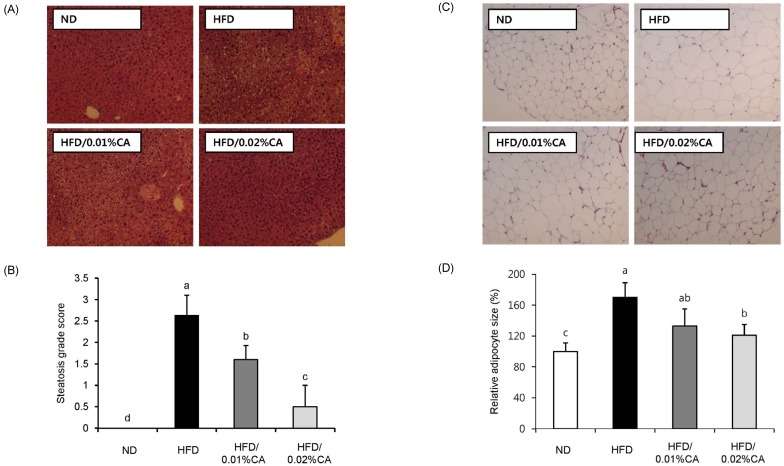
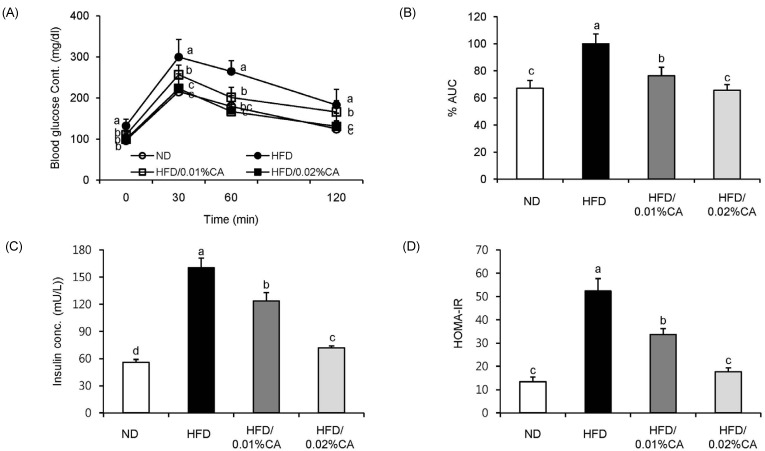
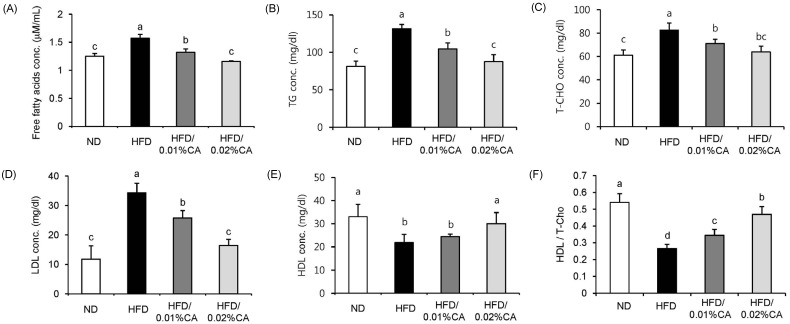
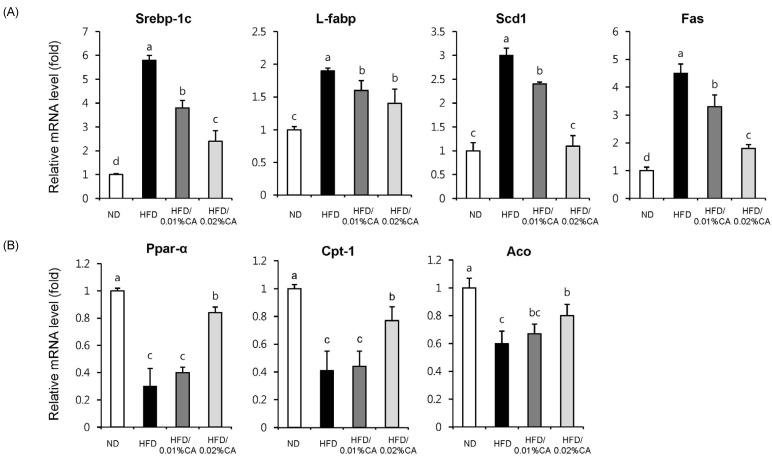
- In this study, we examined the hepatic anti-steatosis activity of carnosic acid (CA), a phenolic compound of rosemary (Rosmarinus officinalis) leaves, as well as its possible mechanism of action, in a high-fat diet (HFD)-fed mice model. Mice were fed a HFD, or a HFD supplemented with 0.01% (w/w) CA or 0.02% (w/w) CA, for a period of 12 weeks, after which changes in body weight, blood lipid profiles, and fatty acid mechanism markers were evaluated. The 0.02% (w/w) CA diet resulted in a marked decline in steatosis grade, as well as in homeostasis model assessment of insulin resistance (HOMA-IR) index values, intraperitoneal glucose tolerance test (IGTT) results, body weight gain, liver weight, and blood lipid levels (P < 0.05). The expression level of hepatic lipogenic genes, such as sterol regulating element binding protein-1c (SREBP-1c), liver-fatty acid binding protein (L-FABP), stearoyl-CoA desaturase 1 (SCD1), and fatty acid synthase (FAS), was significantly lower in mice fed 0.01% (w/w) CA and 0.02% (w/w) CA diets than that in the HFD group; on the other hand, the expression level of beta-oxidation-related genes, such as peroxisome proliferator-activated receptor alpha (PPAR-alpha), carnitine palmitoyltransferase 1 (CPT-1), and acyl-CoA oxidase (ACO), was higher in mice fed a 0.02% (w/w) CA diet, than that in the HFD group (P < 0.05). In addition, the hepatic content of palmitic acid (C16:0), palmitoleic acid (C16:1), and oleic acid (C18:1) was significantly lower in mice fed the 0.02% (w/w) CA diet than that in the HFD group (P < 0.05). These results suggest that orally administered CA suppressed HFD-induced hepatic steatosis and fatty liver-related metabolic disorders through decrease of de novo lipogenesis and fatty acid elongation and increase of fatty acid beta-oxidation in mice.
MeSH Terms
-
Acyl Coenzyme A
Acyl-CoA Oxidase
Animals
Body Weight
Carnitine O-Palmitoyltransferase
Carrier Proteins
Diet
Diet, High-Fat
Diterpenes, Abietane
Fatty Acid Synthetase Complex
Fatty Acids, Monounsaturated
Glucose Tolerance Test
Hand
Homeostasis
Insulin Resistance
Lipogenesis
Liver
Mice
Oleic Acid
Palmitic Acid
Phenol
Plant Extracts
PPAR alpha
Stearoyl-CoA Desaturase
Acyl Coenzyme A
Acyl-CoA Oxidase
Carnitine O-Palmitoyltransferase
Carrier Proteins
Diterpenes, Abietane
Fatty Acid Synthetase Complex
Fatty Acids, Monounsaturated
Oleic Acid
PPAR alpha
Palmitic Acid
Phenol
Plant Extracts
Stearoyl-CoA Desaturase
Figure
Reference
-
1. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010; 51:679–689. PMID: 20041406.
Article2. Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Häkkinen A, Olofsson SO, Yki-Järvinen H, Borén J. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006; 49:755–765. PMID: 16463046.
Article3. Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009; 106:15430–15435. PMID: 19706383.
Article4. Hurjui DM, Niţă O, Graur LI, Mihalache L, Popescu DS, Graur M. The central role of the non alcoholic fatty liver disease in metabolic syndrome. Rev Med Chir Soc Med Nat Iasi. 2012; 116:425–431. PMID: 23077931.5. Yang J, Lv F, Chen XQ, Cui WX, Chen LH, Wen XD, Wang Q. Pharmacokinetic study of major bioactive components in rats after oral administration of extract of Ilex hainanensis by high-performance liquid chromatography/electrospray ionization mass spectrometry. J Pharm Biomed Anal. 2013; 77:21–28. PMID: 23384548.
Article6. Afonso MS, de O Silva AM, Carvalho EB, Rivelli DP, Barros SB, Rogero MM, Lottenberg AM, Torres RP, Mancini-Filho J. Phenolic compounds from Rosemary (Rosmarinus officinalis L.) attenuate oxidative stress and reduce blood cholesterol concentrations in diet-induced hypercholesterolemic rats. Nutr Metab (Lond). 2013; 10:19. PMID: 23374457.
Article7. Manoharan S, Vasanthaselvan M, Silvan S, Baskaran N, Kumar Singh A, Vinoth Kumar V. Carnosic acid: a potent chemopreventive agent against oral carcinogenesis. Chem Biol Interact. 2010; 188:616–622. PMID: 20816777.
Article8. Mengoni ES, Vichera G, Rigano LA, Rodriguez-Puebla ML, Galliano SR, Cafferata EE, Pivetta OH, Moreno S, Vojnov AA. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia. 2011; 82:414–421. PMID: 21129455.
Article9. Tada M, Ohkanda T, Kurabe J. Syntheses of carnosic acid and carnosol, anti-oxidants in Rosemary, from pisiferic acid, the major constituent of Sawara. Chem Pharm Bull (Tokyo). 2010; 58:27–29. PMID: 20045961.
Article10. Wang T, Takikawa Y, Satoh T, Yoshioka Y, Kosaka K, Tatemichi Y, Suzuki K. Carnosic acid prevents obesity and hepatic steatosis in ob/ob mice. Hepatol Res. 2011; 41:87–92. PMID: 21199201.
Article11. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41:1313–1321. PMID: 15915461.
Article12. Lutz SZ, Hennige AM, Feil S, Peter A, Gerling A, Machann J, Kröber SM, Rath M, Schürmann A, Weigert C, Häring HU, Feil R. Genetic ablation of cGMP-dependent protein kinase type I causes liver inflammation and fasting hyperglycemia. Diabetes. 2011; 60:1566–1576. PMID: 21464444.
Article13. Jang HH, Park MY, Kim HW, Lee YM, Hwang KA, Park JH, Park DS, Kwon O. Black rice (Oryza sativa L.) extract attenuates hepatic steatosis in C57BL/6 J mice fed a high-fat diet via fatty acid oxidation. Nutr Metab (Lond). 2012; 9:27. PMID: 22458550.
Article14. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408. PMID: 11846609.15. Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, Micklem KJ, Frayn KN. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009; 52:882–890. PMID: 19252892.
Article16. Wang XA, Deng S, Jiang D, Zhang R, Zhang S, Zhong J, Yang L, Wang T, Hong S, Guo S, She Z, Zhang XD, Li H. CARD3 deficiency exacerbates diet-induced obesity, hepatosteatosis, and insulin resistance in male mice. Endocrinology. 2013; 154:685–697. PMID: 23321697.
Article17. Polyzos SA, Kountouras J, Zavos C. Nonalcoholic fatty liver disease: the pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009; 9:299–314. PMID: 19355912.
Article18. Pettinelli P, Del Pozo T, Araya J, Rodrigo R, Araya AV, Smok G, Csendes A, Gutierrez L, Rojas J, Korn O, Maluenda F, Diaz JC, Rencoret G, Braghetto I, Castillo J, Poniachik J, Videla LA. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta. 2009; 1792:1080–1086. PMID: 19733654.19. Sekiya M, Yahagi N, Matsuzaka T, Najima Y, Nakakuki M, Nagai R, Ishibashi S, Osuga J, Yamada N, Shimano H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003; 38:1529–1539. PMID: 14647064.
Article20. Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int. 2010; 4:741–748. PMID: 21286345.
Article21. Liu X, Strable MS, Ntambi JM. Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Adv Nutr. 2011; 2:15–22. PMID: 22211186.
Article22. Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008; 19:248–256. PMID: 18460915.
Article23. Higuchi N, Kato M, Tanaka M, Miyazaki M, Takao S, Kohjima M, Kotoh K, Enjoji M, Nakamuta M, Takayanagi R. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP and L-FABP in non-alcoholic fatty liver disease. Exp Ther Med. 2011; 2:1077–1081. PMID: 22977624.
Article24. Nagasawa T, Inada Y, Nakano S, Tamura T, Takahashi T, Maruyama K, Yamazaki Y, Kuroda J, Shibata N. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006; 536:182–191. PMID: 16574099.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Genistein Supplementation on Fatty Liver and Lipid Metabolism in Rats Fed High Fat Diet
- Folic acid supplementation prevents high fructose-induced non-alcoholic fatty liver disease by activating the AMPK and LKB1 signaling pathways
- PIK3R3 regulates PPARα expression to stimulate fatty acid β-oxidation and decrease hepatosteatosis
- Korean pine nut oil replacement decreases intestinal lipid uptake while improves hepatic lipid metabolism in mice
- Carnitine Orotate Complex Ameliorates Insulin Resistance and Hepatic Steatosis Through Carnitine Acetyltransferase Pathway