Nutr Res Pract.
2013 Aug;7(4):273-280.
Bamboo salt attenuates CCl4-induced hepatic damage in Sprague-Dawley rats
- Affiliations
-
- 1Department of Food Science and Nutrition, Pusan National University, 30 Jangjun-dong, Geumjung-gu, Busan 609-735, Korea. kunypark@pusan.ac.kr
- 2Department of Biological and Chemical Engineering, Chongqing University of Education, Chongqing 400067, PR China.
Abstract
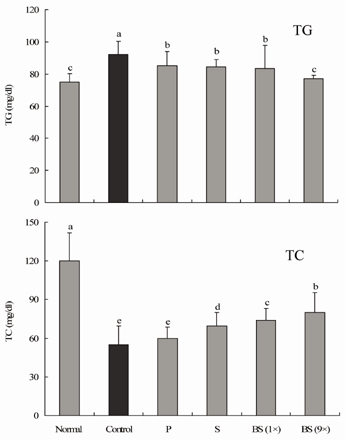
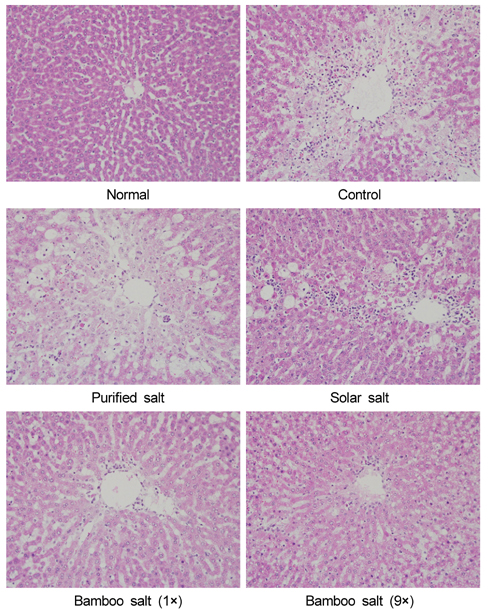
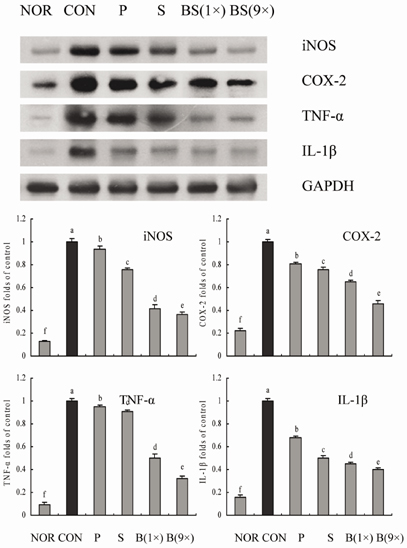
- Bamboo salt, a Korean folk medicine, is prepared with solar salt (sea salt) and baked several times at high temperatures in a bamboo case. In this study, we compared the preventive effects of bamboo salt and purified and solar salts on hepatic damage induced by carbon tetrachloride in Sprague-Dawley rats. Compared with purified and solar salts, bamboo salts prevented hepatic damage in rats, as evidenced by significantly reduced serum levels of aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase (P < 0.05). Bamboo salt (baked 9x) triggered the greatest reduction in these enzyme levels. In addition, it also reduced the levels of the proinflammatory cytokines interleukin (IL)-6, interferon (IFN)-gamma, and tumor necrosis factor (TNF)-alpha. Histopathological sections of liver tissue demonstrated the protective effect of bamboo salt, whereas sections from animals treated with the other salt groups showed a greater degree of necrosis. We also performed reverse transcription-polymerase chain reaction and western blot analyses of the inflammation-related genes iNOS, COX-2, TNF-alpha, and IL-1beta in rat liver tissues. Bamboo salt induced a significant decrease (~80%) in mRNA and protein expression levels of COX-2, iNOS, TNF-alpha, and IL-1beta, compared with the other salts. Thus, we found that baked bamboo salt preparations could prevent CCl4-induced hepatic damage in vivo.
Keyword
MeSH Terms
-
Alanine Transaminase
Animals
Aspartate Aminotransferases
Blotting, Western
Carbon Tetrachloride
Cytokines
Inflammation
Interferons
Interleukins
L-Lactate Dehydrogenase
Liver
Medicine, Traditional
Necrosis
Rats
Rats, Sprague-Dawley
RNA, Messenger
Salts
Tumor Necrosis Factor-alpha
Alanine Transaminase
Aspartate Aminotransferases
Carbon Tetrachloride
Cytokines
Interferons
Interleukins
L-Lactate Dehydrogenase
RNA, Messenger
Salts
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Hu C, Zhang Y, Kitts DD. Evaluation of antioxidant and prooxidant activities of bamboo Phyllostachys nigra var. Henonis leaf extract in vitro. J Agric Food Chem. 2000; 48:3170–3176.
Article2. Shin HY, Na HJ, Moon PD, Seo SW, Shin TY, Hong SH, Lee KN, Park RK, Kim HM. Biological activity of bamboo salt. Food Ind Nutr. 2004; 9:36–45.3. Shin HY, Lee EH, Kim CY, Shin TY, Kim SD, Song YS, Lee KN, Hong SH, Kim HM. Anti-inflammatory activity of Korean folk medicine purple bamboo salt. Immunopharmacol Immunotoxicol. 2003; 25:377–384.
Article4. Cauwels A, Brouckaert P. Survival of TNF toxicity: dependence on caspases and NO. Arch Biochem Biophys. 2007; 462:132–139.
Article5. Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006; 387:349–360.
Article6. Rechnagel RO, Glende EA Jr. Carbon tetrachloride hepatotoxicity: an example of lethal cleavage. CRC Crit Rev Toxicol. 1973; 2:263–297.
Article7. Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003; 33:105–136.
Article8. Raoul JL, Bruix J, Greten TF, Sherman M, Mazzaferro V, Hilgard P, Scherubl H, Scheulen ME, Germanidis G, Dominguez S, Ricci S, Nadel A, Moscovici M, Voliotis D, Llovet JM. Relationship between baseline hepatic status and outcome, and effect of sorafenib on liver function: SHARP trial subanalyses. J Hepatol. 2012; 56:1080–1088.
Article9. Pal M, Ghosh M. Studies on comparative efficacy of α-linolenic acid and α-eleostearic acid on prevention of organic mercury-induced oxidative stress in kidney and liver of rat. Food Chem Toxicol. 2012; 50:1066–1072.
Article10. Small DM, Gobe GC. Cytochrome c: potential as a noninvasive biomarker of drug-induced acute kidney injury. Expert Opin Drug Metab Toxicol. 2012; 8:655–664.
Article11. Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol. 2001; 13:777–784.
Article12. Diehl AM. Cytokine regulation of liver injury and repair. Immunol Rev. 2000; 174:160–171.
Article13. Suliburk JW, Helmer KS, Gonzalez EA, Robinson EK, Mercer DW. Ketamine attenuates liver injury attributed to endotoxemia: role of cyclooxygenase-2. Surgery. 2005; 138:134–140.
Article14. Park CM, Youn HJ, Chang HK, Song YS. TOP1 and 2, polysaccharides from Taraxacum officinale, attenuate CCl4-induced hepatic damage through the modulation of NF-κB and its regulatory mediators. Food Chem Toxicol. 2010; 48:1255–1261.
Article15. Zhao X, Deng X, Park KY, Qiu L, Pang L. Purple bamboo salt has anticancer activity in TCA8113 cells in vitro and preventive effects on buccal mucosa cancer in mice in vivo. Exp Ther Med. 2013; 5:549–554.
Article16. Zhao X, Kim SY, Park KY. Bamboo salt has in vitro anticancer activity in HCT-116 cells and exerts anti-metastatic effects in vivo. J Med Food. 2013; 16:9–19.
Article17. Delić R, Štefanović M. Optimal laboratory panel for predicting preeclampsia. J Matern Fetal Neonatal Med. 2010; 23:96–102.
Article18. Li R, Guo W, Fu Z, Ding G, Zou Y, Wang Z. Hepatoprotective action of Radix Paeoniae Rubra aqueous extract against CCl4-induced hepatic damage. Molecules. 2011; 16:8684–8694.
Article19. Ranawat L, Bhatt J, Patel J. Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylum armatum DC in CCl4 induced hepatic damage in rats. J Ethnopharmacol. 2010; 127:777–780.
Article20. Surendran S, Eswaran MB, Vijayakumar M, Rao CV. In vitro and in vivo hepatoprotective activity of Cissampelos pareira against carbon-tetrachloride induced hepatic damage. Indian J Exp Biol. 2011; 49:939–945.21. Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis. 2006; 49:59–75.
Article22. U.S. Food and Drug Administration [Internet]. Guidance for Industry, Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Silver Spring (MD): U.S. Food and Drug Administration;2005. cited 2013 February 01. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf.23. Raju K, Anbuganapathi G, Gokulakrishnan V, Rajkapoor B, Jayakar B, Manian S. Effect of dried fruits of solanum nigrum LINN against CCl4-induced hepatic damage in rats. Biol Pharm Bull. 2003; 26:1618–1619.
Article24. Segner WP, Schmidt CF, Boltz JK. Effect of sodium chloride and pH on the outgrowth of spores of type E Clostridium botulinum at optimal and suboptimal temperatures. Appl Microbiol. 1966; 14:49–54.
Article25. Zhao X, Jung OS, Park KY. Alkaline and antioxidant effects of bamboo salt. J Korean Soc Food Sci Nutr. 2012; 41:1301–1304.
Article26. Tong Y, Yan D, Gao S. Study on sensitive indexes of acute liver damage induced by carbon tetrachloride. Chin J Food Hyg. 2000; 12:10–11.27. Wang MY, Anderson G, Nowicki D, Jensen J. Hepatic protection by noni fruit juice against CCl4-induced chronic liver damage in female SD rats. Plant Foods Hum Nutr. 2008; 63:141–145.
Article28. Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E. Cholesterol and Recurrent Events (CARE) Investigators. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Circulation. 1998; 98:839–844.
Article29. Gratacós J, Collado A, Filella X, Sanmartí R, Cañete J, Llena J, Molina R, Ballesta A, Muñoz-Gómez J. Serum cytokines (IL-6, TNF-α, IL-1β and IFN-γ) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br J Rheumatol. 1994; 33:927–931.
Article30. Wang JY, Wang XL, Liu P. Detection of serum TNF-α, IFN-γ, IL-6 and IL-8 in patients with hepatitis B. World J Gastroenterol. 1999; 5:38–40.
Article31. Toth B, Yokoyama Y, Schwacha MG, George RL, Rue LW 3rd, Bland KI, Chaudry IH. Insights into the role of interleukin-6 in the induction of hepatic injury after trauma-hemorrhagic shock. J Appl Physiol. 2004; 97:2184–2189.
Article32. Knight B, Lim R, Yeoh GC, Olynyk JK. Interferon-γ exacerbates liver damage, the hepatic progenitor cell response and fibrosis in a mouse model of chronic liver injury. J Hepatol. 2007; 47:826–833.
Article33. Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998; 275:G387–G392.34. Schümann J, Tiegs G. Pathophysiological mechanisms of TNF during intoxication with natural or man-made toxins. Toxicology. 1999; 138:103–126.
Article35. Girish C, Koner BC, Jayanthi S, Rao KR, Rajesh B, Pradhan SC. Hepatoprotective activity of six polyherbal formulations in CCl4-induced liver toxicity in mice. Indian J Exp Biol. 2009; 47:257–263.36. Manjrekar AP, Jisha V, Bag PP, Adhikary B, Pai MM, Hegde A, Nandini M. Effect of Phyllanthus niruri Linn. treatment on liver, kidney and testes in CCl4 induced hepatotoxic rats. Indian J Exp Biol. 2008; 46:514–520.37. Zhao X, Song J, Lee JH, Kim SY, Park KY. Antioxidation and cancer cell (HT-29) antiproliferation effects of Rubus coreanus Miquel bamboo salt. Cancer Prev Res. 2010; 15:306–312.38. Halliwell B. Antioxidants: the basics-what they are and how to evaluate them. Adv Pharmacol. 1997; 38:3–20.
Article39. Zhao X, Kim SH, Qi YC, Kim SY, Park KY. Effects of different kinds of salt in the comutagenicity and growth of cancer cells. J Korean Soc Food Sci Nutr. 2012; 41:26–32.
Article40. Karlsen M, Hovden AO, Vogelsang P, Tysnes BB, Appel S. Bromelain treatment leads to maturation of monocyte-derived dendritic cells but cannot replace PGE2 in a cocktail of IL-1β, IL-6, TNF-α and PGE2. Scand J Immunol. 2011; 74:135–143.
Article41. Nan B, Lin P, Lumsden AB, Yao Q, Chen C. Effects of TNF-α and curcumin on the expression of thrombomodulin and endothelial protein C receptor in human endothelial cells. Thromb Res. 2005; 115:417–426.
Article42. Mills PR, Shenkin A, Anthony RS, McLelland AS, Main AN, MacSween RN, Russell RI. Assessment of nutritional status and in vivo immune responses in alcoholic liver disease. Am J Clin Nutr. 1983; 38:849–859.
Article43. Long RG, Meinhard E, Skinner RK, Varghese Z, Wills MR, Sherlock S. Clinical, biochemical, and histological studies of osteomalacia, osteoporosis, and parathyroid function in chronic liver disease. Gut. 1978; 19:85–90.
Article44. Yunoki H, Mitsuba K, Noma Y, Ishii N, Kawakami H, Kagawa K, Maruhashi A. Magnesium metabolism in liver disease. Gastroenterol Jpn. 1970; 5:70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antifibrotic activity a fermentation filtrate of Ganoderma lucidum
- The Change of Transforming Growth Factor-beta1 Expression and the Effect of Vitamin E in the Acute Rat Liver Injury with Carbon Tetrachloride
- Sparassis crispa Attenuates Carbon Tetrachloride-Induced Hepatic Injury in Rats
- Anti-inflammatory effect of egg white-chalcanthite and purple bamboo salts mixture on arthritis induced by monosodium iodoacetate in Sprague-Dawley rats
- The Expression of G1 Cycins and Rb-E2F, and the Effect of Vitamin E on Hepatic Stellate Cells Activated by CCl4