Nutr Res Pract.
2013 Aug;7(4):262-266.
Laxative effect of peanut sprout extract
- Affiliations
-
- 1School of Food Science and Biotechnology, Kyungpook National University, 80 Daehak-ro, Buk-gu, Daegu 702-701, Korea. vision@knu.ac.kr
- 2Reseacrh Institute for Biological Functions, Chubu University, Aichi 487-8501, Japan.
- 3College of Pharmacy and Research Institute of Pharmaceutical Sciences, Kyungpook National University, Daegu 702-701, Korea.
- 4Huvet Co. Ltd, Iksan, Jeonbuk 570-749, Korea.
Abstract
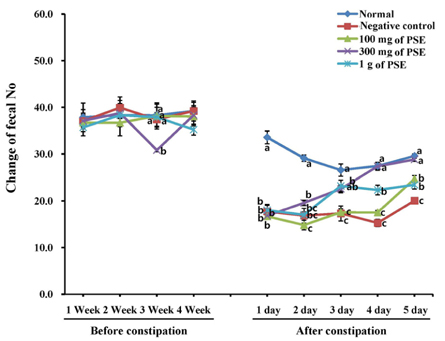
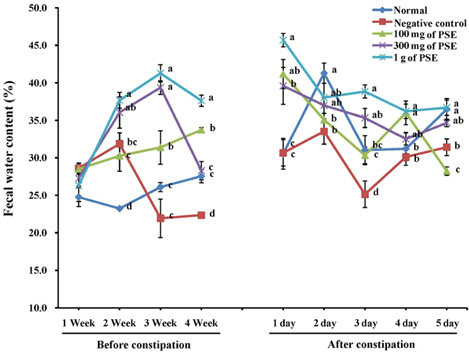
- Certain phenolic compounds are known to exhibit laxative properties. Seed sprouts, such as those of peanut, are known to promote de novo biosynthesis of phenolic compounds. This study was conducted to examine the potential laxative properties of 80% (v/v) ethanolic extract of peanut sprout (PSE), which contains a high concentration of phenolic compounds such as resveratrol. For this, SD rats were orally administered PSE while a control group was incubated with saline. Laxative effects were examined in both groups of rats. Constipation induced by loperamide in SD rats was improved by administration of PSE. Constipated rats showed increased intestinal movement of BaSO4 upon administration of PSE compared to the control, and the groups administered 100 or 1,000 mg PSE/kg bw were not significantly different in transit time of the indicator. However, colon length was not statistically different among the experimental groups, although it was longer in the group incubated with 1 g PSE/kg bw compared to other groups. Further, there was no significant difference in stool number among the experimental groups. Taken together, these findings show that PSE has a laxative effect in a rat model of loperamide-induced constipation.
Keyword
MeSH Terms
Figure
Reference
-
1. Tabbers MM, Boluyt N, Berger MY, Benninga MA. Clinical practice: diagnosis and treatment of functional constipation. Eur J Pediatr. 2011; 170:955–963.2. Loening-Baucke V. Chronic constipation in children. Gastroenterology. 1993; 105:1557–1564.
Article3. Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. 2011; 25:3–18.4. Mostafa SM, Bhandari S, Ritchie G, Gratton N, Wenstone R. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003; 91:815–819.5. Wu Z, Song L, Huang D. Food grade fungal stress on germinating peanut seeds induced phytoalexins and enhanced polyphenolic antioxidants. J Agric Food Chem. 2011; 59:5993–6003.
Article6. Choi JY, Choi DI, Lee JB, Yun SJ, Lee DH, Eun JB, Lee SC. Ethanol extract of peanut sprout induces Nrf2 activation and expression of antioxidant and detoxifying enzymes in human dermal fibroblasts: implication for its protection against UVB-irradiated oxidative stress. Photochem Photobiol. 2013; 89:453–460.
Article7. Ito T, Kakino M, Tazawa S, Watarai T, Oyama M, Maruyama H, Araki Y, Hara H, Iinuma M. Quantification of polyphenols and pharmacological analysis of water and ethanol-based extracts of cultivated agarwood leaves. J Nutr Sci Vitaminol (Tokyo). 2012; 58:136–142.
Article8. Méité S, Bahi C, Yéo D, Datté JY, Djaman JA, N'guessan DJ. Laxative activities of Mareya micrantha (Benth) Müll. Arg. (Euphorbiaceae) leaf aqueous extract in rats. BMC Complement Altern Med. 2010; 10:7.
Article9. Singleton VL, Rossi JA Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965; 16:144–158.10. Chiva-Blanch G, Urpi-Sarda M, Rotchés-Ribalta M, Zamora-Ros R, Llorach R, Lamuela-Raventós RM, Estruch R, Andrés-Lacueva C. Determination of resveratrol and piceid in beer matrices by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2011; 1218:698–705.
Article11. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006; 130:1480–1491.
Article12. Everhart JE, Go VL, Johannes RS, Fitzsimmons SC, Roth HP, White LR. A longitudinal survey of self-reported bowel habits in the United States. Dig Dis Sci. 1989; 34:1153–1162.
Article13. Johanson JF, Sonnenberg A, Koch TR. Clinical epidemiology of chronic constipation. J Clin Gastroenterol. 1989; 11:525–536.
Article14. Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther. 2011; 33:895–901.
Article15. Mugie SM, Di Lorenzo C, Benninga MA. Constipation in childhood. Nat Rev Gastroenterol Hepatol. 2011; 8:502–511.
Article16. Constipation Guideline Committee of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Evaluation and treatment of constipation in infants and children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2006; 43:e1–e13.17. Chmielewska A, Szajewska H. Systematic review of randomised controlled trials: probiotics for functional constipation. World J Gastroenterol. 2010; 16:69–75.18. Burkitt DP, Walker AR, Painter NS. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972; 2:1408–1412.19. Griffenberg L, Morris M, Atkinson N, Levenback C. The effect of dietary fiber on bowel function following radical hysterectomy: a randomized trial. Gynecol Oncol. 1997; 66:417–424.
Article20. Kakino M, Tazawa S, Maruyama H, Tsuruma K, Araki Y, Shimazawa M, Hara H. Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Complement Altern Med. 2010; 10:68.
Article21. Kataoka K, Dimagno EP. Effect of prolonged intraluminal alpha-amylase inhibition on eating, weight, and the small intestine of rats. Nutrition. 1999; 15:123–129.
Article22. Kim JS, Kwon CS, Son KH. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Biosci Biotechnol Biochem. 2000; 64:2458–2461.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peanut sprouts extract (Arachis hypogaea L.) has anti-obesity effects by controlling the protein expressions of PPARgamma and adiponectin of adipose tissue in rats fed high-fat diet
- Peanut sprout ethanol extract inhibits the adipocyte proliferation, differentiation, and matrix metalloproteinases activities in mouse fibroblast 3T3-L1 preadipocytes
- Peanut sprout tea extract inhibits lung metastasis of 4T1 murine mammary carcinoma cells by suppressing the crosstalk between cancer cells and macrophages in BALB/c mice
- Anti-hyperglycemic effects and signaling mechanism of Perilla frutescens sprout extract
- The supplementation effects of peanut sprout on reduction of abdominal fat and health indices in overweight and obese women