Nutr Res Pract.
2013 Jun;7(3):166-171.
Lotus leaf alleviates hyperglycemia and dyslipidemia in animal model of diabetes mellitus
- Affiliations
-
- 1Department of Smart Foods and Drugs, School of Food and Life Science, Inje University, 607 Obang-dong, Gimhae, Gyungnam 621-749, Korea. fdsnkiji@inje.ac.kr
Abstract
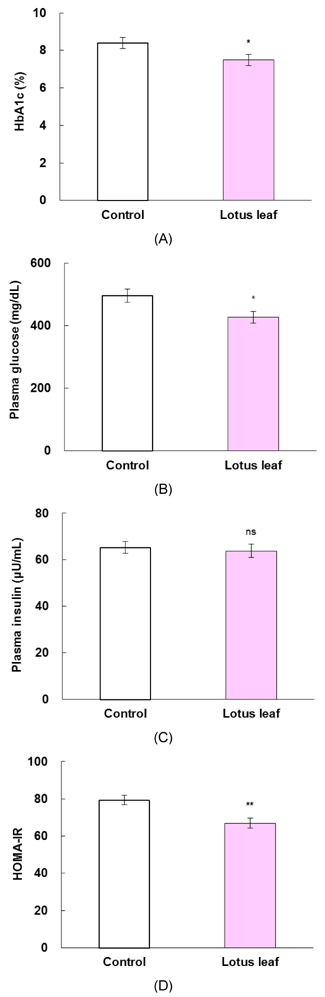
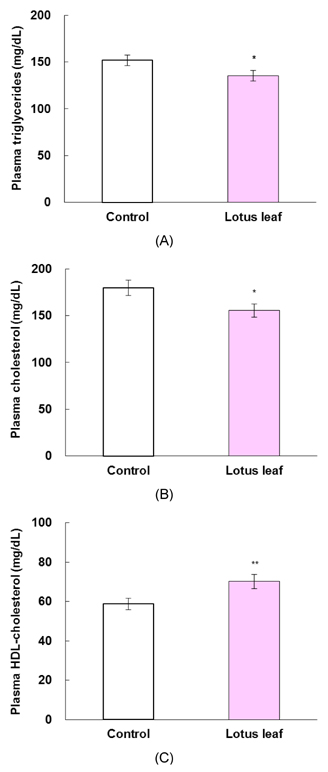
- The purpose of this study was to investigate the effects of lotus leaf on hyperglycemia and dyslipidemia in animal model of diabetes. Inhibitory activity of ethanol extract of lotus leaf against yeast alpha-glucosidase was measured in vitro. The effect of lotus leaf on the postprandial increase in blood glucose levels was assessed in streptozotocin-induced diabetic rats. A starch solution (1 g/kg) with and without lotus leaf extract (500 mg/kg) was administered to the rats after an overnight fast, and postprandial plasma glucose levels were monitored. Four-week-old db/db mice were fed a basal diet or a diet containing 1% lotus leaf extract for 7 weeks after 1 week of acclimation to study the chronic effect of lotus leaf. After sacrifice, plasma glucose, insulin, triglycerides (TG), total cholesterol (CHOL), high-density lipoprotein (HDL)-CHOL, and blood glycated hemoglobin levels were measured. Lotus leaf extract inhibited alpha-glucosidase activity by 37.9%, which was 1.3 times stronger than inhibition by acarbose at a concentration of 0.5 mg/mL in vitro. Oral administration of lotus leaf extract significantly decreased the area under the glucose response curve by 35.1% compared with that in the control group (P < 0.01). Chronic feeding of lotus leaf extract significantly lowered plasma glucose and blood glycated hemoglobin compared with those in the control group. Lotus leaf extract significantly reduced plasma TG and total CHOL and elevated HDL-CHOL levels compared with those in the control group. Therefore, we conclude that lotus leaf is effective for controlling hyperglycemia and dyslipidemia in an animal model of diabetes mellitus.
Keyword
MeSH Terms
-
Acarbose
Acclimatization
Administration, Oral
alpha-Glucosidases
Animals
Blood Glucose
Cholesterol
Diabetes Mellitus
Diet
Dyslipidemias
Ethanol
Glucose
Hemoglobins
Hyperglycemia
Insulin
Lipoproteins
Lotus
Mice
Models, Animal
Plasma
Rats
Starch
Triglycerides
Yeasts
Acarbose
Blood Glucose
Cholesterol
Ethanol
Glucose
Hemoglobins
Insulin
Lipoproteins
Starch
Triglycerides
alpha-Glucosidases
Figure
Reference
-
1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998; 21:1414–1431.
Article2. Garg A, Grundy SM. Management of dyslipidemia in NIDDM. Diabetes Care. 1990; 13:153–169.
Article3. American Diabetes Association. Summary of revisions for the 2008 clinical practice recommendations. Diabetes Care. 2008; 31:S3–S4.4. American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care. 1998; 21:179–182.5. Standl E, Baumgartl HJ, Füchtenbusch M, Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes Metab. 1999; 1:215–220.
Article6. Sels JP, Huijberts MS, Wolffenbuttel BH. Miglitol, a new alphaglucosidase inhibitor. Expert Opin Pharmacother. 1999; 1:149–156.7. Hanefeld M. The role of acarbose in the treatment of noninsulin-dependent diabetes mellitus. J Diabetes Complications. 1998; 12:228–237.
Article8. Mai TT, Thu NN, Tien PG, Van Chuyen N. Alpha-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J Nutr Sci Vitaminol (Tokyo). 2007; 53:267–276.
Article9. Gonzalez M, Zarzuelo A, Gamez MJ, Utrilla MP, Jimenez J, Osuna I. Hypoglycemic activity of olive leaf. Planta Med. 1992; 58:513–515.
Article10. Wang H, Du YJ, Song HC. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010; 123:6–13.
Article11. Sridhar KR, Bhat R. Lotus - a potential nutraceutical source. J Agric Technol. 2007; 3:143–155.12. Mukherjee PK, Mukherjee D, Maji AK, Rai S, Heinrich M. The sacred lotus (Nelumbo nucifera) - phytochemical and therapeutic profile. J Pharm Pharmacol. 2009; 61:407–422.
Article13. Zhou T, Luo D, Li XY, Luo Y. Hypoglycemic and hypolipidemic effects of flavonoids from lotus (Nelumbo nuficera Gaertn) leaf in diabetic mice. J Med Plant Res. 2009; 3:290–293.14. Lee SK, Hwang JY, Song JH, Jo JR, Kim MJ, Kim ME, Kim JI. Inhibitory activity of Euonymus alatus against alphaglucosidase in vitro and in vivo. Nutr Res Pract. 2007; 1:184–188.
Article15. Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of α-glucosidase inhibitors from tochu-cha (Eucommia ulmoides). Biosci Biotechnol Biochem. 1997; 61:177–178.
Article16. Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi S, Farhangi A, Verdi AA, Mofidian SM, Rad BL. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007; 22:60–64.
Article17. Raabo E, Terkildsen TC. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960; 12:402–407.
Article18. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993; 123:1939–1951.
Article19. Grossman SH, Mollo E, Ertingshausen G. Simplified, totally enzymatic method for determination of serum triglycerides with a centrifugal analyzer. Clin Chem. 1976; 22:1310–1313.
Article20. Kattermann R, Jaworek D, Möller G, Assmann G, Björkhem I, Svensson L, Borner K, Boerma G, Leijnse B, Desager JP, Harwengt C, Kupke I, Trinder P. Multicentre study of a new enzymatic method of cholesterol determination. J Clin Chem Clin Biochem. 1984; 22:245–251.
Article21. Wadehra NR. A colorimetric method for the estimation of cholesterol from high density lipoprotein and its subclasses. Indian J Clin Biochem. 1990; 5:131–134.
Article22. Schifreen RS, Hickingbotham JM, Bowers GN Jr. Accuracy, precision, and stability in measurement of hemoglobin A1c by "high-performance" cation-exchange chromatography. Clin Chem. 1980; 26:466–472.
Article23. Morgan CR, Lazarow A. Immunoassay of insulin: two antibody system, plasma insulin levels in normal, subdiabetic and diabetic rats. Diabetes. 1963; 12:115–126.
Article24. Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997; 20:1087–1092.
Article25. Lin HY, Kuo YH, Lin YL, Chiang W. Antioxidative effect and active components from leaves of Lotus (Nelumbo nucifera). J Agric Food Chem. 2009; 57:6623–6629.
Article26. Matsui T, Tanaka T, Tamura S, Toshima A, Tamaya K, Miyata Y, Tanaka K, Matsumoto K. alpha-Glucosidase inhibitory profile of catechins and theaflavins. J Agric Food Chem. 2007; 55:99–105.27. Jo SH, Ka EH, Lee HS, Apostolidis E, Jang HD, Kwon YI. Comparison of antioxidant potential and rat intestinal α-glucosidases inhibitory activities of quercetin, rutin, and isoquercetin. Int J Appl Res Nat Prod. 2009; 2:52–60.28. Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011; 5:107–111.
Article29. Abrahamson MJ. Optimal glycemic control in type 2 diabetes mellitus: fasting and postprandial glucose in context. Arch Intern Med. 2004; 164:486–491.
Article30. Wajchenberg BL. Postprandial glycemia and cardiovascular disease in diabetes mellitus. Arq Bras Endocrinol Metabol. 2007; 51:212–221.
Article31. Lebovitz HE. α-Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Rev. 1998; 6:132–145.32. Huang CF, Chen YW, Yang CY, Lin HY, Way TD, Chiang W, Liu SH. Extract of lotus leaf (Nelumbo nucifera) and its active constituent catechin with insulin secretagogue activity. J Agric Food Chem. 2011; 59:1087–1094.
Article33. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002; 48:436–472.
Article34. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403.
Article35. O'Keefe JH Jr, Miles JM, Harris WH, Moe RM, McCallister BD. Improving the adverse cardiovascular prognosis of type 2 diabetes. Mayo Clin Proc. 1999; 74:171–180.36. Taskinen MR. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003; 46:733–749.
Article37. Brown WV, Clark L, Falko JM, Guyton JR, Rees TJ, Schonfeld G, Lopes-Virella MF. Optimal management of lipids in diabetes and metabolic syndrome. J Clin Lipidol. 2008; 2:335–342.
Article38. Guérin M, Le Goff W, Lassel TS, Van Tol A, Steiner G, Chapman MJ. Atherogenic role of elevated CE transfer from HDL to VLDL(1) and dense LDL in type 2 diabetes : impact of the degree of triglyceridemia. Arterioscler Thromb Vasc Biol. 2001; 21:282–288.39. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.
Article40. Lee MS, Kim CT, Kim Y. Green tea (-)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann Nutr Metab. 2009; 54:151–157.
Article41. Kobori M, Masumoto S, Akimoto Y, Oike H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol Nutr Food Res. 2011; 55:530–540.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk Factors Management in Diabetic Patients
- Postprandial Hyperglycemia
- Early Diagnosis of Diabetes Mellitus
- A Case of Type 2 Diabetes Mellitus Initially Presented as Monochorea Associated with Ketotic Hyperglycemia
- Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice