Nutr Res Pract.
2012 Dec;6(6):499-504.
Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3T3-L1 preadipocytes
- Affiliations
-
- 1Department of Food and Nutrition, Eulji University, Seongnam, Gyunggi 461-723, Korea.
- 2Department of Food Science and Nutrition, Dankook University, 126 Jukjeon-dong, Suji-gu, Yongin-si, Gyunggi 448-701, Korea. wkkim@dankook.ac.kr
Abstract
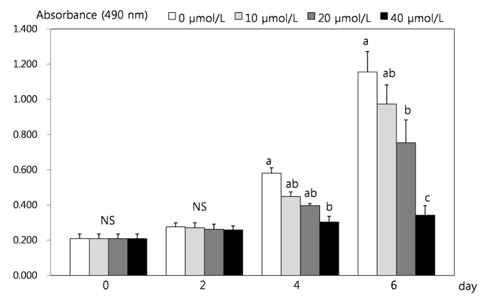
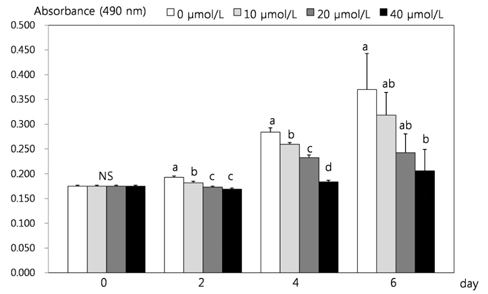
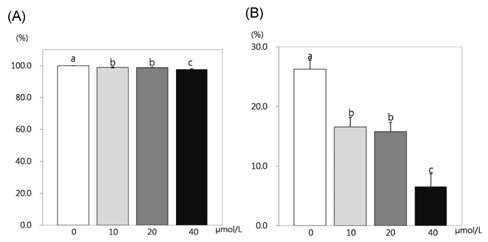
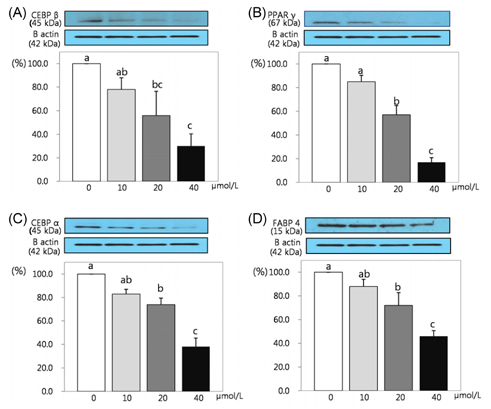
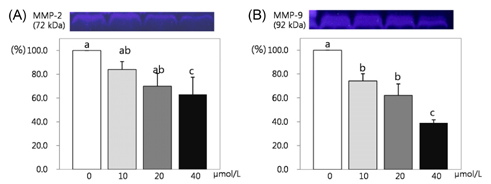
- This study attempted to investigate the effects of resveratrol on the differentiation of adipocytes. After cells were treated with various concentrations of resveratrol (0, 10, 20, and 40 micromol/L), adipocyte proliferation, the protein expression of transcription factors, and MMPs' activities were determined. Cell proliferation was inhibited more within 4 days of incubation (P < 0.05), and lipid accumulation in adipocyte was significantly inhibited by 93.8%, 92.4% and 91.5%, respectively, after two days of 10, 20, and 40 micromol/L resveratrol treatment (P < 0.05). Six days of incubation with the three resveratrol concentrations caused a significantly decreases of 63%, 59.9%, and 25.1% GPDH activity as a dose-dependent response. The triglyceride concentration also decreased significantly with the increase of resveratrol concentration (P < 0.05). The protein expression of CCAAT/enhancer-binding protein (C/EBPbeta) was decreased significantly by 56% and 30% while PPARgamma was significantly reduced by 57% and 15% with resveratrol treatments of 20 and 40 micromol/L, respectively (P < 0.05). The protein expression of C/EBPalpha was decreased by 83%, 74%, and 38% to increased dosage levels, with significance determined for this decrease from 20 micromol/L of resveratrol. The protein expression of fatty acid binding protein (FABP4) was decreased significantly by 88%, 72%, and 46% with the increase of resveratrol concentration. The activity of MMP-2 was decreased significantly by 84%, 70%, and 63% while MMP-9 activity was decreased significantly by 74%, 62%, and 39% with the increased resveratrol concentrations of 10, 20, and 40 micromol/L, respectively (P < 0.05).
MeSH Terms
Figure
Reference
-
1. World Health Organization. International Association for the Study of Obesity. International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. 2000. Sydney: Health Communications;15–21.2. Ministry of Health and Welfare. National Health and Nutrition Examination Survey Report 2010. 2010. Seoul: Ministry of Health and Welfare.3. Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008. 453:783–787.
Article4. Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991. 5:1538–1552.
Article5. Distel RJ, Robinson GS, Spiegelman BM. Fatty acid regulation of gene expression. Transcriptional and post-transcriptional mechanisms. J Biol Chem. 1992. 267:5937–5941.
Article6. Björntorp P. Fat cell distribution and metabolism. Ann N Y Acad Sci. 1987. 499:66–72.
Article7. Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997. 4:211–232.
Article8. de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans. 2007. 35:1156–1160.
Article9. Levi F, Pasche C, Lucchini F, Ghidoni R, Ferraroni M, La Vecchia C. Resveratrol and breast cancer risk. Eur J Cancer Prev. 2005. 14:139–142.
Article10. Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the 'French paradox'? Eur J Endocrinol. 1998. 138:619–620.
Article11. Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci. 2002. 957:210–229.
Article12. Ray PS, Maulik G, Cordis GA, Bertelli AA, Bertelli A, Das DK. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic Biol Med. 1999. 27:160–169.
Article13. Das S, Alagappan VK, Bagchi D, Sharma HS, Maulik N, Das DK. Coordinated induction of iNOS-VEGF-KDR-eNOS after resveratrol consumption: a potential mechanism for resveratrol preconditioning of the heart. Vascul Pharmacol. 2005. 42:281–289.14. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006. 444:337–342.
Article15. Chen S, Li Z, Li W, Shan Z, Zhu W. Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via activation of AMPK. Can J Physiol Pharmacol. 2011. 89:793–799.16. Szkudelska K, Nogowski L, Szkudelski T. Resveratrol, a naturally occurring diphenolic compound, affects lipogenesis, lipolysis and the antilipolytic action of insulin in isolated rat adipocytes. J Steroid Biochem Mol Biol. 2009. 113:17–24.
Article17. Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975. 5:19–27.
Article18. Lee MS, Kim CT, Kim CJ, Cho YJ, Kim Y. Effects of Portulaca oleracea L. extract on lipolysis and hormone sensitive lipase (HSL) gene expression in 3T3-L1 adipocytes. Korean J Nutr. 2006. 39:742–747.19. Kim MJ, Kim Y, Chung JH, Kim JW, Kim HK. The effect of caffeine on 3T3-L1 adipocyte differentiation : a nutrigenomical approach. Korean J Nutr. 2005. 38:649–655.20. Chon JW, Sung JH, Hwang EJ, Park YK. Chlorella methanol extract reduces lipid accumulation in and increases the number of apoptotic 3T3-L1 cells. Ann N Y Acad Sci. 2009. 1171:183–189.
Article21. Tomiyama K, Nakata H, Sasa H, Arimura S, Nishio E, Watanabe Y. Wortmannin, a specific phosphatidylinositol 3-kinase inhibitor, inhibits adipocytic differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 1995. 212:263–269.
Article22. Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem. 1979. 254:273–275.
Article23. Vankoningsloo S, De Pauw A, Houbion A, Tejerina S, Demazy C, de Longueville F, Bertholet V, Renard P, Remacle J, Holvoet P, Raes M, Arnould T. CREB activation induced by mitochondrial dysfunction triggers triglyceride accumulation in 3T3-L1 preadipocytes. J Cell Sci. 2006. 119:1266–1282.
Article24. Na MH, Seo EY, Kim WK. Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells. Nutr Res Pract. 2009. 3:265–271.
Article25. Kwon SY, Kang KJ. The effect of conjugated linoleic acid isomers on the cell proliferation, apotosis and expressions of uncoupling protein (Ucp) genes during differentiation of 3T3-L1 preadipocytes. Korean J Nutr. 2004. 37:533–539.26. Park HJ, Yang JY, Ambati S, Della-Fera MA, Hausman DB, Rayalam S, Baile CA. Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes. J Med Food. 2008. 11:773–783.
Article27. Rayalam S, Yang JY, Ambati S, Della-Fera MA, Baile CA. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res. 2008. 22:1367–1371.
Article28. Cho KJ, Moon HE, Moini H, Packer L, Yoon DY, Chung AS. Alpha-lipoic acid inhibits adipocyte differentiation by regulating pro-adipogenic transcription factors via mitogen-activated protein kinase pathways. J Biol Chem. 2003. 278:34823–34833.
Article29. Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996. 15:5336–5348.
Article30. Zhang XH, Huang B, Choi SK, Seo JS. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutr Res Pract. 2012. 6:286–293.
Article31. Yang JY, Della-Fera MA, Rayalam S, Ambati S, Hartzell DL, Park HJ, Baile CA. Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008. 82:1032–1039.
Article32. Jeong YS, Jung HK, Cho KH, Youn KS, Hong JH. Anti-obesity effect of grape skin extract in 3T3-L1 adipocytes. Food Sci Biotechnol. 2011. 20:635–642.
Article33. Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, Song L. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life Sci. 2006. 78:2564–2570.
Article34. Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L, Sun Y, Zhang Z, Zhu W. Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J Nutr Biochem. 2012. 23:1100–1112.
Article35. Hajra AK, Larkins LK, Das AK, Hemati N, Erickson RL, MacDougald OA. Induction of the peroxisomal glycerolipid-synthesizing enzymes during differentiation of 3T3-L1 adipocytes. Role in triacylglycerol synthesis. J Biol Chem. 2000. 275:9441–9446.
Article36. McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001. 13:534–540.
Article37. Bouloumié A, Sengenès C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001. 50:2080–2086.38. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998. 78:783–809.
Article39. Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol. 2001. 152:693–703.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Peanut sprout ethanol extract inhibits the adipocyte proliferation, differentiation, and matrix metalloproteinases activities in mouse fibroblast 3T3-L1 preadipocytes
- Cydonia oblonga Miller fruit extract exerts an anti-obesity effect in 3T3-L1 adipocytes by activating the AMPK signaling pathway