Nutr Res Pract.
2012 Aug;6(4):301-307.
Effect of quercetin on impaired immune function in mice exposed to irradiation
- Affiliations
-
- 1Division of Biological Science, College of Science, Sookmyung Women's University, Seoul 140-742, Korea.
- 2Major in Food and Nutrition, College of Human Ecology, Sookmyung Women's University, Chungpa-dong 2ga, Yongsan-gu, Seoul 140-742, Korea. hskim@sookmyung.ac.kr
Abstract
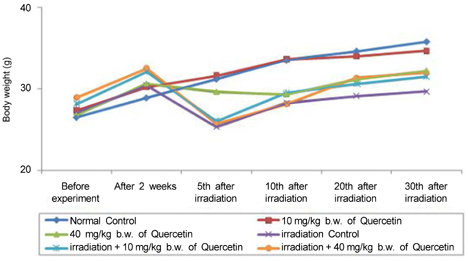
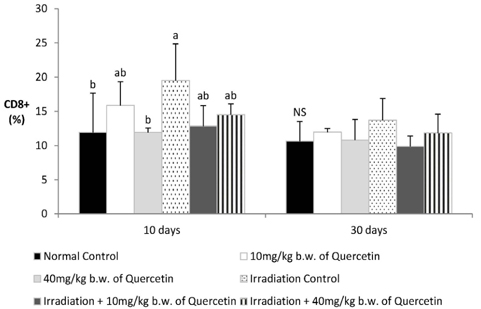
- Radiation used in cancer treatment may cause side effects such as inflammation. Quercetin is a polyphenol that reduces inflammation. This study evaluated the recovery efficacy of quercetin on impaired immune function in irradiation-induced inflammatory mice. Quercetin administered at two concentrations of 10 and 40 mg/kg body weight was initiated 2 weeks before irradiation and was continued 30 days after irradiation. The animals exposed/not exposed to radiation were sacrificed on radiation days 10 and 30. Splenocyte proliferation, which was diminished after irradiation, was enhanced significantly by quercetin supplementation after 30 days of irradiation. Cytokine secretion increased in the radiation group compared to that in the non-radiation control group. After 30 days of radiation, interleukin (IL)-1beta and IL-6 secretion decreased significantly in the radiation-quercetin groups. When quercetin was administered for 44 days, it showed a possible protective effect against irradiation-induced inflammation in mice. Quercetin could be beneficial in the recovery of irradiation-induced increases in cytokine secretion.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim SH, Jo SK, Kwon OD. The radioprotective effects of rhizomata herbs. J Radiat Prot. 2001. 26:13–18.2. Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer. 1993. 20:21–29.
Article3. Sharafetdinov KhKh, Plotnikova OA, Voznyĭ EK. Nutrition management of the cancer patients: modern view on problem. Vopr Pitan. 2008. 77:4–14.4. Mojzisová G, Kuchta M. Dietary flavonoids and risk of coronary heart disease. Physiol Res. 2001. 50:529–535.5. Giugliano D. Dietary antioxidants for cardiovascular prevention. Nutr Metab Cardiovasc Dis. 2000. 10:38–44.6. Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am J Clin Nutr. 2005. 81:268S–276S.
Article7. Heo BG, Chon SU, Park YJ, Bae JH, Park SM, Park YS, Jang HG, Gorinstein S. Antiproliferative activity of Korean wild vegetables on different human tumor cell lines. Plant Foods Hum Nutr. 2009. 64:257–263.
Article8. Bors W, Saran M. Radical scavenging by flavonoid antioxidants. Free Radic Res Commun. 1987. 2:289–294.
Article9. Gavelová M, Hladíková J, Vildová L, Novotná R, Vondrácek J, Krcmár P, Machala M, Skálová L. Reduction of doxorubicin and oracin and induction of carbonyl reductase in human breast carcinoma MCF-7 cells. Chem Biol Interact. 2008. 176:9–18.
Article10. Cai Q, Rahn RO, Zhang R. Dietary flavonoids, quercetin, luteolin and genistein, reduce oxidative DNA damage and lipid peroxidation and quench free radicals. Cancer Lett. 1997. 119:99–107.
Article11. Avila MA, Velasco JA, Cansado J, Notario V. Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res. 1994. 54:2424–2428.12. Nagata H, Takekoshi S, Takeyama R, Homma T, Yoshiyuki Osamura R. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment Cell Res. 2004. 17:66–73.
Article13. Garabige V, Giraud P, De Rycke Y, Girod A, Jouffroy T, Jaulerry C, Brunin F, Rodriguez J. Impact of nutrition management in patients with head and neck cancers treated with irradiation: is the nutritional intervention useful? Cancer Radiother. 2007. 11:111–116.14. Kamrava M, Bernstein MB, Camphausen K, Hodge JW. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the Three Musketeers or just another quixotic combination? Mol Biosyst. 2009. 5:1262–1270.
Article15. Toda M, Wang L, Ogura S, Torii M, Kurachi M, Kakimi K, Nishikawa H, Matsushima K, Shiku H, Kuribayashi K, Kato T. UV irradiation of immunized mice induces type 1 regulatory T cells that suppress tumor antigen specific cytotoxic T lymphocyte responses. Int J Cancer. 2011. 129:1126–1136.
Article16. Gaton DD, Lichter H, Avisar I, Slodovinic D, Solomon AS. Lymphocytic reaction to ultraviolet radiation on rabbit conjunctiva. Ann Ophthalmol (Skokie). 2007. 39:128–133.
Article17. Sgadari C, Angiolillo AL, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996. 87:3877–3882.
Article18. Gwak HS, Yoo HJ, Youn SM, Lee DH, Kim MS, Rhee CH. Radiosurgery for recurrent brain metastases after whole-brain radiotherapy : factors affecting radiation-induced neurological dysfunction. J Korean Neurosurg Soc. 2009. 45:275–283.
Article19. Rao V, Chaukar D, D'Cruz AK. Hypercalcemia and treated breast cancers: the diagnostic dilemma. J Cancer Res Ther. 2009. 5:46–48.
Article20. Barros GC, Labate RC. Psychological repercussions related to brachytherapy treatment in women with gynecological cancer: analysis of production from 1987 to 2007. Rev Lat Am Enfermagem. 2008. 16:1049–1053.
Article21. Ghadjar P, Vock J, Vetterli D, Manser P, Bigler R, Tille J, Madlung A, Behrensmeier F, Mini R, Aebersold DM. Acute and late toxicity in prostate cancer patients treated by dose escalated intensity modulated radiation therapy and organ tracking. Radiat Oncol. 2008. 3:35.
Article22. Lee YR, Jung JH, Kim HS. Hesperidin partially restores impaired immune and nutritional function in irradiated mice. J Med Food. 2011. 14:475–482.
Article23. Kim KO, Chun M, Kang S, Kim HS. Effect of high protein diet and resveratrol supplementation on the nutritional status and immunoreactivity in the irradiation-induced inflammatory rats. Korean J Nutr. 2009. 42:605–614.
Article24. Mishell BB, Shiigi SM. Selected Methods in Cellular Immunology. 1980. San Francisco: Freeman.25. Gosselin TK, Gilliard L, Tinnen R. Assessing the need for a dietitian in radiation oncology. Clin J Oncol Nurs. 2008. 12:781–787.
Article26. Bhanja P, Saha S, Kabarriti R, Liu L, Roy-Chowdhury N, Roy-Chowdhury J, Sellers RS, Alfieri AA, Guha C. Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS One. 2009. 4:e8014.
Article27. Nagai H, Nishiyori T, Ochi T, Imai Y, Tanaka H, Inagaki N. The effect of methanolic extract from Corydalis Tuber on cytokine production and allergic reactions in experimental animals. J Tradit Med. 1999. 16:51–57.28. Peter W. Understanding Immunology. 2006. 2nd edition. Harlow, England: Pearson Prentice Hall;98–110.29. Taylor CW, Nisbet A, McGale P, Darby SC. Cardiac exposures in breast cancer radiotherapy: 1950s-1990s. Int J Radiat Oncol Biol Phys. 2007. 69:1484–1495.
Article30. Yokoyama Y, Sakamoto K, Arai M, Akagi M. Radiation and surgical stress induce a significant impairment in cellular immunity in patients with esophageal cancer. Jpn J Surg. 1989. 19:535–543.
Article31. Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009. 9:15–27.
Article32. Osada J, Kamocki Z, Rusak M, Dabrowska M, Kedra B. The effect of surgical and nutritional treatment on activation parameters of peripheral blood T lymphocytes in stomach cancer patients in postoperative period. Pol Merkur Lekarski. 2008. 24:231–236.33. Jiang W, Kang L, Lu HZ, Pan X, Lin Q, Pan Q, Xue Y, Weng X, Tang YW. Normal values for CD4 and CD8 lymphocyte subsets in healthy Chinese adults from Shanghai. Clin Diagn Lab Immunol. 2004. 11:811–813.
Article34. Nagel JE, Chopra RK, Chrest FJ, McCoy MT, Schneider EL, Holbrook NJ, Adler WH. Decreased proliferation, interleukin 2 synthesis, and interleukin 2 receptor expression are accompanied by decreased mRNA expression in phytohemagglutinin-stimulated cells from elderly donors. J Clin Invest. 1988. 81:1096–1102.
Article35. Rechciński T, Grebowska A, Kurpesa M, Sztybrych M, Peruga JZ, Trzos E, Rudnicka W, Krzemińska-Pakuła M, Chmiela M. Interleukin-1b and interleukin-1 receptor inhibitor gene cluster polymorphisms in patients with coronary artery disease after percutaneous angioplasty or coronary artery bypass grafting. Kardiol Pol. 2009. 67:601–610.36. Meng Z, Liu Y, Wu D. Effect of sulfur dioxide inhalation on cytokine levels in lungs and serum of mice. Inhal Toxicol. 2005. 17:303–307.
Article37. Meydani SN. Dietary modulation of cytokine production and biologic functions. Nutr Rev. 1990. 48:361–369.
Article38. Wu KS, Zhou X, Zheng F, Xu XQ, Lin YH, Yang J. Influence of interleukin-1 beta genetic polymorphism, smoking and alcohol drinking on the risk of non-small cell lung cancer. Clin Chim Acta. 2010. 411:1441–1446.
Article39. Hepgül G, Tanrikulu S, Unalp HR, Akguner T, Erbil Y, Olgaç V, Ademoğlu E. Preventive effect of pentoxifylline on acute radiation damage via antioxidant and anti-inflammatory pathways. Dig Dis Sci. 2010. 55:617–625.
Article40. Calveley VL, Khan MA, Yeung IW, Vandyk J, Hill RP. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol. 2005. 81:887–899.
Article41. Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005. 9:Suppl 2. S51–S63.
Article42. Isenring EA, Bauer JD, Capra S. Nutrition support using the American Dietetic Association medical nutrition therapy protocol for radiation oncology patients improves dietary intake compared with standard practice. J Am Diet Assoc. 2007. 107:404–412.
Article43. Rockenbach G, Di Pietro PF, Ambrosi C, Boaventura BC, Vieira FG, Crippa CG, Da Silva EL, Fausto MA. Dietary intake and oxidative stress in breast cancer: before and after treatments. Nutr Hosp. 2011. 26:737–744.44. Kröner A, Stoll H, Spichiger E. Malnutrition and weight lossnurse assessment of nutritional status and counselling: experiences of patients with newly diagnosed or relapsed cancer. Pflege. 2012. 25:85–95.
Article45. Noroozi M, Burns J, Crozier A, Kelly IE, Lean ME. Prediction of dietary flavonol consumption from fasting plasma concentration or urinary excretion. Eur J Clin Nutr. 2000. 54:143–149.
Article46. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008. 22:659–661.
Article47. Ghosh A, Mandal AK, Sarkar S, Panda S, Das N. Nanoencapsulation of quercetin enhances its dietary efficacy in combating arsenic-induced oxidative damage in liver and brain of rats. Life Sci. 2009. 84:75–80.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Inhibitory Effect of Quercetin on Adipose Tissue Inflammation in Mice Fed on a High-fat Diet
- Effect of Sound Stress on Immune Response
- Effect of quercetin on the anti-tumor activity of cisplatin in EMT6 breast tumor-bearing mice
- Study on the Recovery of Epidermal Langerhans Cells in C3H Mice after UVA Irradiation
- Failure of immunization with Naegleria fowleri in mice born to immune mothers