Nutr Res Pract.
2012 Jun;6(3):187-194.
Antibacterial effect of citrus press-cakes dried by high speed and far-infrared radiation drying methods
- Affiliations
-
- 1School of Marine Biomedical Sciences, Jeju National University, 1 Ara-dong, Jeju-si, Jeju 690-756, Korea. youjinj@jejunu.ac.kr
- 2Marine Bioprocess Research Centre, Pukyoung National University, Busan 608-737, Korea.
- 3Food Biotechnology Major, Kunsan National University, Jeonbuk 573-701, Korea.
- 4Department of Food Science and Nutrition, Pukyoung National University, Busan 608-737, Korea.
- 5Department of Tourism Hotel Culinary Art, Jeju College of Technology, Jeju 690-140, Korea.
Abstract
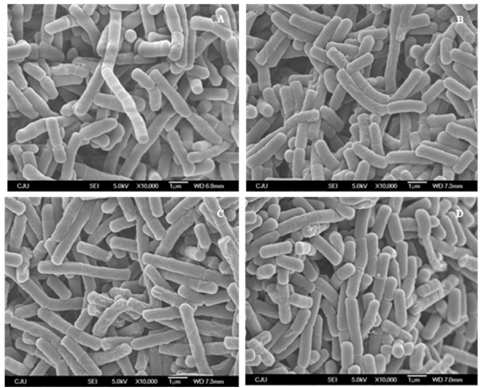
- In this study, the antibacterial effect was evaluated to determine the benefits of high speed drying (HSD) and far-infrared radiation drying (FIR) compared to the freeze drying (FD) method. Citrus press-cakes (CPCs) are released as a by-product in the citrus processing industry. Previous studies have shown that the HSD and FIR drying methods are much more economical for drying time and mass drying than those of FD, even though FD is the most qualified drying method. The disk diffusion assay was conducted, and the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined with methanol extracts of the dried CPCs against 11 fish and five food-related pathogenic bacteria. The disk diffusion results indicated that the CPCs dried by HSD, FIR, and FD prevented growth of all tested bacteria almost identically. The MIC and MBC results showed a range from 0.5-8.0 mg/mL and 1.0-16.0 mg/mL respectively. Scanning electron microscopy indicated that the extracts changed the morphology of the bacteria cell wall, leading to destruction. These results suggest that CPCs dried by HSD and FIR showed strong antibacterial activity against pathogenic bacteria and are more useful drying methods than that of the classic FD method in CPCs utilization.
Keyword
MeSH Terms
Figure
Reference
-
1. Cabello FC. Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol. 2006. 8:1137–1144.
Article2. Alzoreky NS, Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int J Food Microbiol. 2003. 80:223–230.
Article3. Kim JS, Kim Y. The inhibitory effect of natural bioactives on the growth of pathogenic bacteria. Nutr Res Pract. 2007. 1:273–278.
Article4. Dorman HJ, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000. 88:308–316.
Article5. Abu-Shanab B, Adwan G, Abu-Safiya D, Jarrar N, Adwan K. Antibacterial activities of some plant extracts utilized in popular medicine in Palestine. Turk J Biol. 2004. 28:99–102.6. Sakai M. Current research status of fish immunostimulants. Aquaculture. 1999. 172:63–92.
Article7. Verschuere L, Rombaut G, Sorgeloos P, Verstraete W. Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev. 2000. 64:655–671.
Article8. Ortuño A, Báidez A, Gómez P, Arcas MC, Porras I, García-Lidón A, Del Río JA. Citrus paradisi and Citrus sinensis Flavonoids: Their influence in the defence mechanism against Penicillium digitatum. Food Chem. 2006. 98:351–358.
Article9. Yi ZB, Yu Y, Liang YZ, Zeng B. In vitro antioxidant and antimicrobial activities of the extract of Pericarpium Citri Reticulatae of a new Citrus cultivar and its main flavonoids. Lebenson Wiss Technol. 2007. 41:597–603.
Article10. Jo C, Park BJ, Chung SH, Kim CB, Cha BS, Byun MW. Antibacterial and anti-fungal activity of citrus (Citrus unshiu) essential oil extracted from peel by-products. Food Sci Biotechnol. 2004. 13:384–386.11. Kitzberger CS, Smânia A Jr, Pedrosa RC, Ferreira SR. Antioxidant and antimicrobial activities of shiitake (Lentinula edodes) extracts obtained by organic solvents and supercritical fluids. J Food Eng. 2007. 80:631–638.
Article12. Huang B, Aung SK, Wang Y. Thousand Formulas and Thousand Herbs of Traditional Chinese Medicine. 1993. vol. 1. Harbin: Heilongjiang Education Press.13. Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci. 2009. 22:277–281.14. Johann S, Oliveira VL, Pizzolatti MG, Schripsema J, Braz-Filho R, Branco A, Smânia A Jr. Antimicrobial activity of wax and hexane extracts from Citrus spp. peels. Mem Inst Oswaldo Cruz. 2007. 102:681–685.
Article15. Yao X, Pan S, Duan C, Yang F, Fan G, Zhu X, Yang S, Xu X. Polymethoxylated flavone extracts from citrus peel for use in the functional food and nutraceutical industry. Food Sci Biotechnol. 2009. 18:1237–1242.16. Sun Y, Wang J, Gu S, Liu Z, Zhang Y, Zhang X. Simultaneous determination of flavonoids in different parts of Citrus reticulata 'Chachi' fruit by high performance liquid chromatography-photodiode array detection. Molecules. 2010. 15:5378–5388.
Article17. Choi I, Choi S, Ji J. Flavonoids and functional properties of germinated citron (Citrus junos Sieb. ex TANAKA) shoots. Food Sci Biotechnol. 2009. 18:1224–1229.18. Gorinstein S, Martín-Belloso O, Park YS, Haruenkit R, Lojek A, Ĉíž M, Caspi A, Libman I, Trakhtenberg S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001. 74:309–315.
Article19. Mahmud S, Saleem M, Siddique S, Ahmed R, Khanum R, Perveen Z. Volatile components, antioxidant and antimicrobial activity of Citrus acida var. sour lime peel oil. J Saudi Chem Soc. 2009. 13:195–198.
Article20. Matasyoh JC, Kiplimo JJ, Karubiu NM, Hailstorks TP. Chemical composition and antimicrobial activity of essential oil of Tarchonanthus camphorates. Food Chem. 2007. 101:1183–1187.
Article21. Zhang M, Zhang JP, Ji HT, Wang JS, Qian DH. Effect of six flavonoids on proliferation of hepatic stellate cells in vitro. Acta Pharmacol Sin. 2000. 21:253–256.22. Lee SW, Najiah M. Antimicrobial property of 2-hydroxypropane-1,2,3-Tricarboxylic acid isolated from Citrus microcarpa extract. Agric Sci China. 2009. 8:880–886.
Article23. Nannapaneni R, Muthaiyan A, Crandall PG, Johnson MG, O'Bryan CA, Chalova VI, Callaway TR, Carroll JA, Arthington JD, Nisbet DJ, Ricke SC. Antimicrobial activity of commercial citrus-based natural extracts against Escherichia coli O157:H7 isolates and mutant strains. Foodborne Pathog Dis. 2008. 5:695–699.
Article24. Senevirathne M, Jeon YJ, Ha JH, Kim SH. Effective drying of citrus by-product by high speed drying: A novel drying technique and their antioxidant activity. J Food Eng. 2009. 92:157–163.
Article25. Senevirathne M, Kim SH, Kim YD, Oh CK, Oh MC, Ahn CB, Je JY, Lee WW, Jeon YJ. Effect of far-infrared radiation drying of citrus press-cakes on free radical scavenging and antioxidant activities. J Food Eng. 2010. 97:168–176.
Article26. Senevirathne M, Kim SH, Um BH, Lee JS, Ha JH, Lee WW, Jeon YJ. Effect of high speed drying on antioxidant properties of enzymatic digests from citrus by-products and their protective effect on DNA damage induced by H2O2. Food Sci Biotechnol. 2009. 18:672–681.27. Callaway TR, Carroll JA, Edrington TS, Anderson RC, Collier CT, Nisbet DJ. Watson R, Gerald JL, Preedy VR, editors. Citrus products and their use against bacteria: Potential health and cost benefits. Nutrients, Dietary Supplements, and Nutriceutical: Cost Analysis Versus Clinical Benefits. 2011. New York: Humana press;277–286.
Article28. Senevirathne M, Jeon YJ, Ha JH, Kim SH. Effect of far-infrared radiation for dying citrus by-products and their radical scavenging activities and protective effects against H2O2 - induced DNA damage. J Food Sci Nutr. 2008. 13:313–320.
Article29. Meena MR, Sethi V. Antimicrobial activity of essential oils from spices. J Food Sci Technol. 1994. 31:68–70.30. Rota C, Carramiñana JJ, Burillo J, Herrera A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J Food Prot. 2004. 67:1252–1256.
Article31. Cai L, Wu CD. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J Nat Prod. 1996. 59:987–990.
Article32. Patrick R. Antimicrobial Agents and Susceptibility Testing. 1996. Washington, DC: Murray.33. Bae EA, Han MJ, Kim DH. In vitro anti-Helicobacter pylori activity of some flavonoids and their metabolites. Planta Med. 1999. 65:442–443.
Article34. Taguri T, Tanaka T, Kouno I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol Pharm Bull. 2004. 27:1965–1969.
Article35. Scherrer R, Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971. 107:718–735.
Article36. Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985. 49:1–32.
Article37. Denyer SP, Hugo WB. Denyer SP, Hugo WB, editors. Mechanisms of antibacterial action - a summary. Mechanisms of Action of Chemical Biocides. 1991. Oxford: Blackwell Scientific Publications;331–334.38. Kim YS, Kim HH, Yoo MJ, Shin DH. Bactericidal effect of the extracts of Polygonum cuspidatum on Bacillus cereus. Food Sci Biotechnol. 2004. 13:430–433.39. Nychas GJE. Gould GW, editor. Natural antimicrobials from plants. New Methods of Food Preservation. 1995. London: Blackie Academic and Professional;58–89.
Article40. de Billerbeck VG, Roques CG, Bessière JM, Fonvieille JL, Dargent R. Effects of Cymbopogon nardus (L.) W. Watson essential oil on the growth and morphogenesis of Aspergillus niger. Can J Microbiol. 2001. 47:9–17.
Article41. Tsuchiya H, Iinuma M. Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine. 2000. 7:161–165.
Article42. Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta. 1993. 1147:132–136.
Article43. Ultee A, Bennik MH, Moezelaar R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 2002. 68:1561–1568.
Article44. Beuchat LR, Golden DA. Antimicrobials occurring naturally in foods. Food Technol. 1989. 43:134–142.45. Haraguchi H, Tanimoto K, Tamura Y, Mizutani K, Kinoshita T. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry. 1998. 48:125–129.
Article46. Mori A, Nishino C, Enoki N, Tawata S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry. 1998. 26:2231–2234.
Article47. Ohemeng KA, Schwender CF, Fu KP, Barrett JF. DNA gyrase inhibitory and antibacterial activity of some flavones (1). Bioorg Med Chem Lett. 1993. 3:225–230.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective effect of methanol extract from citrus press cakes prepared by far-infrared radiation drying on H2O2-mediated oxidative damage in Vero cells
- The effect of temperature and storage time on DNA integrity after freeze-drying sperm from individuals with normozoospermia
- Effect of moisture and drying time on the bond strength of the one-step self-etching adhesive system
- Experimental study on production of Freeze Dried B.C.G. Vaccine in Korea
- A Study on Freeze - Dried Bone