Nutr Res Pract.
2011 Dec;5(6):533-539.
Coconut-derived D-xylose affects postprandial glucose and insulin responses in healthy individuals
- Affiliations
-
- 1Department of Food and Nutritional Sciences, Hanbuk University, Gyeonggi 483-777, Korea.
- 2Department of Food and Nutrition, Sookmyung Women's University, 100 Chungpa-ro 47-gil, Yongsan-gu, Seoul 140-742, Korea. mksung@sm.ac.kr
- 3Foods R&D, CJ Cheiljedang Co., Seoul 150-050, Korea.
Abstract
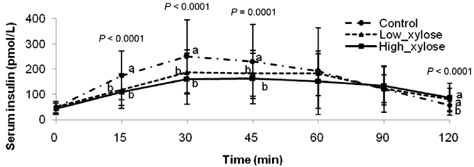
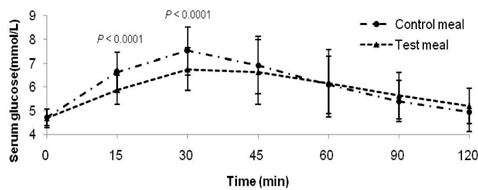
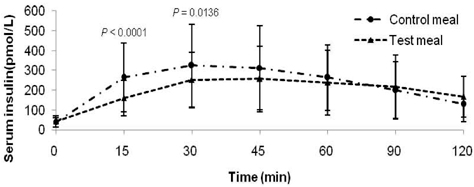
- Metabolic alterations including postprandial hyperglycemia have been implicated in the development of obesity-related diseases. Xylose is a sucrase inhibitor suggested to suppress the postprandial glucose surge. The objectives of this study were to assess the inhibitory effects of two different concentrations of xylose on postprandial glucose and insulin responses and to evaluate its efficacy in the presence of other macronutrients. Randomized double-blind cross-over studies were conducted to examine the effect of D-xylose on postprandial glucose and insulin response following the oral glucose tolerance test (OGTT). In study 1, the overnight-fasted study subjects (n = 49) consumed a test sucrose solution (50 g sucrose in 130 ml water) containing 0, 5, or 7.5 g D-xylose powder. In study 2, the overnight-fasted study subjects (n = 50) consumed a test meal (50 g sucrose in a 60 g muffin and 200 ml sucrose-containing solution). The control meal provided 64.5 g of carbohydrates, 4.5 g of fat, and 10 g of protein. The xylose meal was identical to the control meal except 5 g of xylose was added to the muffin mix. In study 1, the 5 g xylose-containing solutions exhibited significantly lower area under the glucose curve (AUCg) and area under the insulin curve (AUCi) values for 0-15 min (P < 0.0001, P < 0.0001), 0-30 min (P < 0.0001, P < 0.0001), 0-45 min (P < 0.0001, P < 0.0001), 0-60 min (P < 0.0001, P < 0.0001), 0-90 min (P < 0.0001, P < 0.0001) and 0-120 min (P = 0.0071, P = 0.0016). In study 2, the test meal exhibited significantly lower AUCg and AUCi values for 0-15 min (P < 0.0001, P < 0.0001), 0-30 min (P < 0.0001, P < 0.0001), 0-45 min (P < 0.0001, P = 0.0005), 0-60 min (P = 0.0002, P = 0.0025), and 0-90 min (P = 0.0396, P = 0.0246). In conclusion, xylose showed an acute suppressive effect on the postprandial glucose and insulin surges.
MeSH Terms
Figure
Reference
-
1. Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007. 91:1063–1077. viii
Article2. Wojtusciszyn A, Armanet M, Morel P, Berney T, Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia. 2008. 51:1843–1852.
Article3. Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981. 34:362–366.
Article4. Salmerón J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997. 277:472–477.
Article5. Tucci SA, Boyland EJ, Halford JC. The role of lipid and carbohydrate digestive enzyme inhibitors in the management of obesity: a review of current and emerging therapeutic agents. Diabetes Metab Syndr Obes. 2010. 3:125–143.
Article6. McDougall GJ, Kulkarni NN, Stewart D. Current developments on the inhibitory effects of berry polyphenols on digestive enzymes. Biofactors. 2008. 34:73–80.
Article7. Minami Y, Kuriyama C, Ikeda K, Kato A, Takebayashi K, Adachi I, Fleet GW, Kettawan A, Okamoto T, Asano N. Effect of five-membered sugar mimics on mammalian glycogen-degrading enzymes and various glucosidases. Bioorg Med Chem. 2008. 16:2734–2740.
Article8. Seri K, Sanai K, Matsuo N, Kawakubo K, Xue C, Inoue S. L-arabinose selectively inhibits intestinal sucrase in an uncompetitive manner and suppresses glycemic response after sucrose ingestion in animals. Metabolism. 1996. 45:1368–1374.
Article9. Asano T, Yoshmura Y, Kunugita K. Sucrase inhibitory activity of D-xylose and effect on the elevation of blood glucose in rats. J Jpn Soc Nutr Food Sci. 1996. 49:157–162.
Article10. Gruzman A, Shamni O, Ben Yakir M, Sandovski D, Elgart A, Alpert E, Cohen G, Hoffman A, Katzhendler Y, Cerasi E, Sasson S. Novel D-xylose derivatives stimulate muscle glucose uptake by activating AMP-activated protein kinase alpha. J Med Chem. 2008. 51:8096–8108.
Article11. Gannon MC, Nuttall FQ. Control of blood glucose in type 2 diabetes without weight loss by modification of diet composition. Nutr Metab (Lond). 2006. 3:16.
Article12. Leeman M, Ostman E, Björck I. Vinegar dressing and cold storage of potatoes lowers postprandial glycaemic and insulinaemic responses in healthy subjects. Eur J Clin Nutr. 2005. 59:1266–1271.
Article13. Frauchiger MT, Wenk C, Colombani PC. Effects of acute chromium supplementation on postprandial metabolism in healthy young men. J Am Coll Nutr. 2004. 23:351–357.
Article14. Williams JA, Lai CS, Corwin H, Ma Y, Maki KC, Garleb KA, Wolf BW. Inclusion of guar gum and alginate into a crispy bar improves postprandial glycemia in humans. J Nutr. 2004. 134:886–889.
Article15. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972. 18:499–502.
Article16. Wolever TM, Jenkins DJ, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991. 54:846–854.
Article17. Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000. 85:2970–2973.
Article18. Gavin JR 3rd. Pathophysiologic mechanisms of postprandial hyperglycemia. Am J Cardiol. 2001. 88:4H–8H.
Article19. Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2001. 17:164–174.
Article20. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002. 287:2414–2423.21. Clapp JF, Lopez B. Low-Versus High-Glycemic Index Diets in Women: Effects on Caloric Requirement, Substrate Utilization and Insulin Sensitivity. Metab Syndr Relat Disord. 2007. 5:231–242.
Article22. Oh K, Hu FB, Cho E, Rexrode KM, Stampfer MJ, Manson JE, Liu S, Willett WC. Carbohydrate intake, glycemic index, glycemic load, and dietary fiber in relation to risk of stroke in women. Am J Epidemiol. 2005. 161:161–169.
Article23. Beulens JW, de Bruijne LM, Stolk RP, Peeters PH, Bots ML, Grobbee DE, van der Schouw YT. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol. 2007. 50:14–21.
Article24. Hodge AM, English DR, O'Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004. 27:2701–2706.
Article25. Krog-Mikkelsen I, Hels O, Tetens I, Holst JJ, Andersen JR, Bukhave K. The effects of L-arabinose on intestinal sucrase activity: dose-response studies in vitro and in humans. Am J Clin Nutr. 2011. 94:472–478.
Article26. Shibanuma K, Degawa Y, Houda K. Determination of the transient period of the EIS complex and investigation of the suppression of blood glucose levels by L-arabinose in healthy adults. Eur J Nutr. 2011. 50:447–453.
Article27. Lodefalk M, Aman J, Bang P. Effects of fat supplementation on glycaemic response and gastric emptying in adolescents with Type 1 diabetes. Diabet Med. 2008. 25:1030–1035.
Article28. Preuss HG, Echard B, Bagchi D, Stohs S. Inhibition by natural dietary substances of gastrointestinal absorption of starch and sucrose in rats and pigs: 1. Acute studies. Int J Med Sci. 2007. 4:196–202.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Nutrient Preload and Food Order on Glucose, Insulin, and Gut Hormones
- Diet Therapy for Postprandial Hyperglycemia in Patients with Diabetes
- Clinical Meaning of Postprandial Insulin Secretory Function in Korean Type 2 Diabetes Mellitus
- D-Xylose as a sugar complement regulates blood glucose levels by suppressing phosphoenolpyruvate carboxylase (PEPCK) in streptozotocin-nicotinamide-induced diabetic rats and by enhancing glucose uptake in vitro
- Postprandial hypoglycemic effect of mulberry leaf in Goto-Kakizaki rats and counterpart control Wistar rats