Nutr Res Pract.
2011 Dec;5(6):503-510.
The anti-obesity effect of Lethariella cladonioides in 3T3-L1 cells and obese mice
- Affiliations
-
- 1Department of Medical Nutrition, Graduate School of East-West Medical Science, Kyung Hee University, 1 Seocheon-dong, Giheung-gu, Yongin, Gyeonggi 446-701, Korea. ypark@khu.ac.kr
- 2Department of Endocrinology and Metabolism, Kyung Hee University School of Medicine, Seoul 130-701, Korea.
- 3Research Institute of Medical Nutrition, Kyung Hee University, Seoul 130-701, Korea.
Abstract
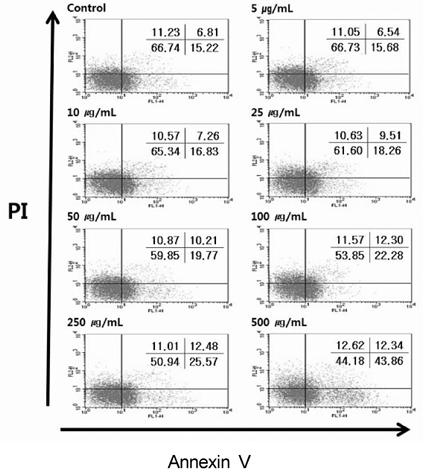
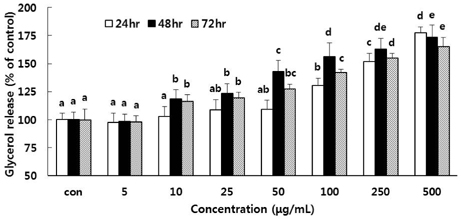
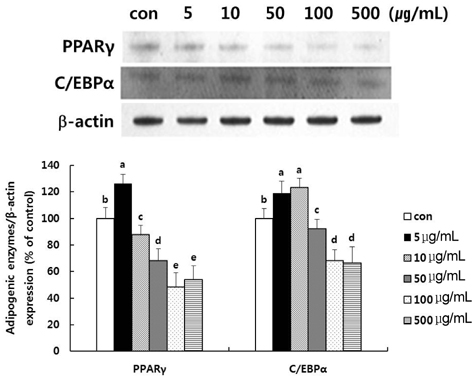
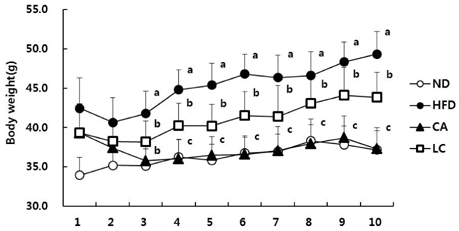
- The aim of this study was to investigate whether a water extract of L. cladonioides (LC) has an anti-obesity effect in 3T3-L1 cells and obese mice. Treatment of differentiated 3T3-L1 adipocytes with LC caused a significant increase in glycerol release and reduced the protein expression of the adipogenic transcription factors, PPARgamma and C/EBPalpha. In an animal model, obese mice were artificially induced by a high fat diet for 10 weeks. Experimental groups were treated with LC (100 mg/kg/day) by gavage for the next 10 weeks. At the end of experiment, the body weight of the LC group mice was reduced by 14.2% compared to the high fat diet (HFD) group. The treatment also decreased liver (31.0%), epididymal (18.0%) and retroperitoneal (19.3%) adipose tissue, and kidney (6.7%) weights, respectively, compared with those of the HFD group. LC prevented diet-induced increases in the serum level of TC (22.6%), TG (11.6%), and glucose (35.0%), respectively, compared with the HFD group. However, the HDL-C level was higher in the LC group (26.1%) than the HFD group. The results of this study thus suggest that LC suppressed lipid accumulation and expression of adipogenic transcription factors, and increased the amount of glycerol release. LC also indicated an anti-obese and anti-hyperlipidemic effect.
Keyword
MeSH Terms
Figure
Reference
-
1. Poulos SP, Dodson MV, Hausman GJ. Cell line models for differentiation: preadipocytes and adipocytes. Exp Biol Med (Maywood). 2010. 235:1185–1193.
Article2. Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2007. 13:3540–3553.
Article3. Lee WJ, Koh EH, Won JC, Kim MS, Park JY, Lee KU. Obesity: the role of hypothalamic AMP-activated protein kinase in body weight regulation. Int J Biochem Cell Biol. 2005. 37:2254–2259.
Article4. Kersten S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001. 2:282–286.
Article5. Shi Y, Burn P. Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nat Rev Drug Discov. 2004. 3:695–710.
Article6. Chen N, Bezzina R, Hinch E, Lewandowski PA, Cameron-Smith D, Mathai ML, Jois M, Sinclair AJ, Begg DP, Wark JD, Weisinger HS, Weisinger RS. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr Res. 2009. 29:784–793.
Article7. Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004. 50:1511–1525.
Article8. Park YS, Yoon Y, Ahn HS. Platycodon grandiflorum extract represses up-regulated adipocyte fatty acid binding protein triggered by a high fat feeding in obese rats. World J Gastroenterol. 2007. 13:3493–3499.
Article9. Pittler MH, Schmidt K, Ernst E. Adverse events of herbal food supplements for body weight reduction: systematic review. Obes Rev. 2005. 6:93–111.
Article10. Naik GH, Priyadarsini KI, Satav JG, Banavalikar MM, Sohoni DP, Biyani MK, Mohan H. Comparative antioxidant activity of individual herbal components used in Ayurvedic medicine. Phytochemistry. 2003. 63:97–104.
Article11. Slanc P, Doljak B, Kreft S, Lunder M, Janes D, Strukelj B. Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytother Res. 2009. 23:874–877.
Article12. Saito M, Ueno M, Ogino S, Kubo K, Nagata J, Takeuchi M. High dose of Garcinia cambogia is effective in suppressing fat accumulation in developing male Zucker obese rats, but highly toxic to the testis. Food Chem Toxicol. 2005. 43:411–419.
Article13. Obermayer W. On the identity of lethariella sinensis wei & jiang, with new reports of tibetan lethariella species. Bibl Lichenol. 2001. 78:321–326.14. Jiang B, Zhao QS, Yang H, Hou AJ, Lin ZW, Sun HD. Constituents from Lethariella cladonioides. Fitoterapia. 2001. 72:832–833.15. Kinoshita K, Togawa T, Hiraishi A, Nakajima Y, Koyama K, Narui T, Wang LS, Takahashi K. Antioxidant activity of red pigments from the lichens Lethariella sernanderi, L. cashmeriana, and L. sinensis. J Nat Med. 2010. 64:85–88.
Article16. Niu DL, Harada H, Wang LS, Zhang YJ, Yang CR. Chemotaxonomic study of the Lethariella cladonioides complex (lichenized Ascomycota, Parmeliaceae). Lichenologist. 2011. 43:213–223.
Article17. Ramírez-Zacarías JL, Castro-Muñozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992. 97:493–497.
Article18. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957. 226:497–509.
Article19. Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun. 2010. 11:145–156.
Article20. Moussalli C, Downs RW, May JM. Potentiation by glucose of lipolytic responsiveness of human adipocytes. Diabetes. 1986. 35:759–763.
Article21. Hauck M, Jürgens SR, Willenbruch K, Huneck S, Leuschner C. Dissociation and metal-binding characteristics of yellow lichen substances suggest a relationship with site preferences of lichens. Ann Bot. 2009. 103:13–22.
Article22. Cowherd RM, Lyle RE, McGehee RE Jr. Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999. 10:3–10.
Article23. Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res. 2006. 50:176–187.
Article24. Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, Lyons TJ. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J Am Coll Nutr. 2010. 29:31–40.
Article25. Shehzad A, Ha T, Subhan F, Lee YS. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur J Nutr. 2011. 50:151–161.
Article26. Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond). 2005. 29:Suppl 1. S13–S16.27. Fajas L, Fruchart JC, Auwerx J. Transcriptional control of adipogenesis. Curr Opin Cell Biol. 1998. 10:165–173.
Article28. Chun MR, Lee YJ, Kim KH, Kim YW, Park SY, Lee KM, Kim JY, Park YK. Differential effects of high-carbohydrate and high-fat diet composition on muscle insulin resistance in rats. J Korean Med Sci. 2010. 25:1053–1059.
Article29. Hursel R, Westerterp-Plantenga MS. Thermogenic ingredients and body weight regulation. Int J Obes (Lond). 2010. 34:659–669.
Article30. Zheng G, Sayama K, Okubo T, Juneja LR, Oguni I. Anti-obesity effects of three major components of green tea, catechins, caffeine and theanine, in mice. In Vivo. 2004. 18:55–62.31. Yun JW, Shin ES, Cho SY, Kim SH, Kim CW, Lee TR, Kim BH. The effects of BADGE and caffeine on the time-course response of adiponectin and lipid oxidative enzymes in high fat diet-fed C57BL/6J mice: correlation with reduced adiposity and steatosis. Exp Anim. 2008. 57:461–469.
Article32. Murosaki S, Lee TR, Muroyama K, Shin ES, Cho SY, Yamamoto Y, Lee SJ. A combination of caffeine, arginine, soy isoflavones, and L-carnitine enhances both lipolysis and fatty acid oxidation in 3T3-L1 and HepG2 cells in vitro and in KK mice in vivo. J Nutr. 2007. 137:2252–2257.
Article33. Mori S, Satou M, Kanazawa S, Yoshizuka N, Hase T, Tokimitsu I, Takema Y, Nishizawa Y, Yada T. Body fat mass reduction and up-regulation of uncoupling protein by novel lipolysis-promoting plant extract. Int J Biol Sci. 2009. 5:311–318.
Article34. Jung RT, Shetty PS, James WP, Barrand MA, Callingham BA. Caffeine: its effect on catecholamines and metabolism in lean and obese humans. Clin Sci (Lond). 1981. 60:527–535.
Article35. Chumark P, Khunawat P, Sanvarinda Y, Phornchirasilp S, Morales NP, Phivthong-Ngam L, Ratanachamnong P, Srisawat S, Pongrapeeporn KU. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J Ethnopharmacol. 2008. 116:439–446.
Article36. Wei AH, Zhou DN, Ruan JL, Cai YL, Xiong CM, Li MX. Characterisation of phenols and antioxidant and hypolipidaemic activities of Lethariella cladonioides. J Sci Food Agric. 2012. 92:373–379.
Article37. Frayn KN, Maycock PF. Skeletal muscle triacylglycerol in the rat: methods for sampling and measurement, and studies of biological variability. J Lipid Res. 1980. 21:139–144.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice
- Nelumbo nucifera Leaf Extract Regulates Lipid Metabolism and Differentiation in 3T3-L1 Adipocytes and db/db Mice
- Expression of eotaxin in 3T3-L1 adipocytes and the effects of weight loss in high-fat diet induced obese mice
- Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3