Nutr Res Pract.
2011 Oct;5(5):435-442.
A mixture of Salacia oblonga extract and IP-PA1 reduces fasting plasma glucose (FPG) and low-density lipoprotein (LDL) cholesterol levels
- Affiliations
-
- 1Department of Nutritional Science, Okayama Prefectural University, 111 Kuboki, Soja, 719-1197, Japan.
- 2Non-profit Organization, Linking Setouchi Innate Immune Network, 388-1 Satozuka-azanishi, Kami-ita-cho, Itano, Tokushima 771-1342, Japan.
- 3Department of Integrated and Holistic Immunology, Faculty of Medicine, Kagawa University, 1750-1 Oaza-ikenobe, Miki-cho, Kida-gun, Kagawa 761-0793, Japan. sma5628@tokushima.bunri-u.ac.jp
- 4Central Park Clinic, 1-10-16 Ban-cho, Takamatsu, Kagawa 760-0017, Japan.
- 5Nakagawa Hospital, 2-17-17 Mukaishin-machi, Minami-ku, Fukuoka 811-1345, Japan.
- 6Miyake Medical Institute, 1-10-16 Ban-cho, Takamatsu, Kagawa 760-0017, Japan.
- 7Faculty of Medicine, School of Medicine, Fukuoka University, 8-19-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan.
- 8Institute for Health and Science, Tokushima Bunri University, Nishihama, Yamashiro-cho, Tokushima, Tokushima 770-8514, Japan.
Abstract
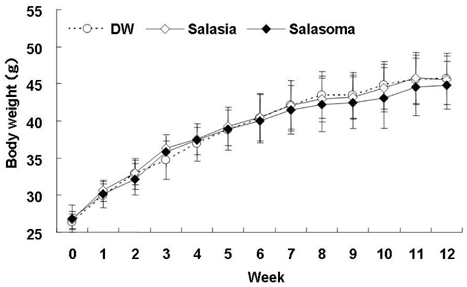
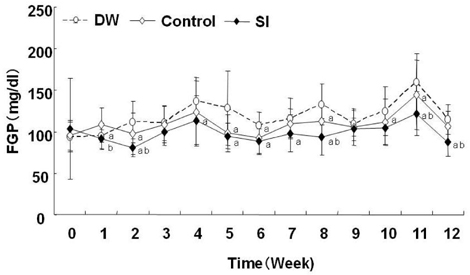
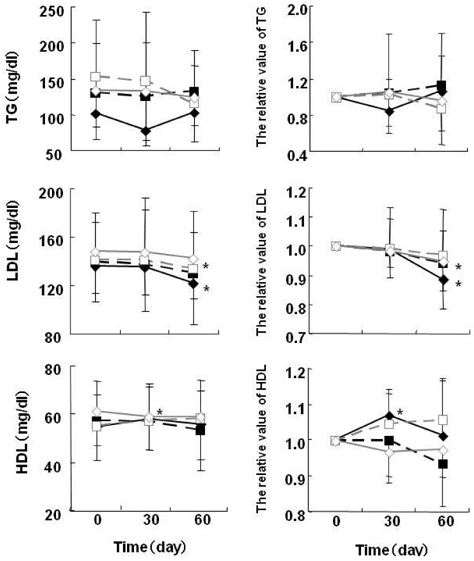
- At present, lifestyle-related diseases are one of the most critical health issues worldwide. It has been reported that lipopolysaccharide derived from a Gram-negative bacteria (IP-PA1) symbiotic with wheat exhibited several advantageous biological effects, such as the reduction of plasma glucose levels in NOD mice and low-density lipoprotein (LDL) levels in WHHL rabbits. In this study, the beneficial effects on plasma glucose and lipids of a tea (SI tea) consisting of IP-PA1 and Salacia (which contains an inhibitor of alpha-glucosidase) were investigated in the KK-Ay/TaJcl type 2 diabetic model mice and in human subjects with premetabolic syndrome in a double-blind, randomized study. SI tea significantly decreased plasma glucose levels in KK-Ay/TaJcl mice. A clinical trial of SI tea was performed with 41 subjects between the ages of 40 and 69, who belonged either to a high plasma glucose group (HG: FPG 100-125 mg/dl) or to a hyperlipidemia group (HL: TG > or = 150 mg/dl, or LDL > or = 120 mg/dl, or HDL < 40 mg/dl). These subjects ingested either Salacia without IP-PA1 (the control) or SI tea. Blood samples were collected at 0, 30, and 60 days after initiating SI tea treatment, and were measured for FPG, HbA1c, TG, LDL, and HDL. These results showed that SI tea reduced FPG and HbA1c more rapidly than the control in the HL group, and also significantly improved LDL and HDL levels in the HG group. Thus, SI tea may be helpful in preventing lifestyle-related diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004. 27:1047–1053.
Article2. Adachi M, Yamaoka K, Watanabe M, Nishikawa M, Hida E, Kobayashi I, Tango T. Effects of lifestyle education program for type 2 diabetes patients in clinics: study design of a cluster randomized trial. BMC Public Health. 2010. 10:742.
Article3. Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006. 2:367–373.
Article4. Wolf BW, Weisbrode SE. Safety evaluation of an extract from Salacia oblonga. Food Chem Toxicol. 2003. 41:867–874.
Article5. Yoshikawa M, Morikawa T, Matsuda H, Tanabe G, Muraoka O. Absolute stereostructure of potent alpha-glucosidase inhibitor, Salacinol, with unique thiosugar sulfonium sulfate inner salt structure from Salacia reticulata. Bioorg Med Chem. 2002. 10:1547–1554.
Article6. Ghavami A, Johnston BD, Pinto BM. A new class of glycosidase inhibitor: synthesis of salacinol and its stereoisomers. J Org Chem. 2001. 66:2312–2317.
Article7. Yoshikawa M, Murakami T, Yashiro K, Matsuda H. Kotalanol, a potent alpha-glucosidase inhibitor with thiosugar sulfonium sulfate structure, from antidiabetic ayurvedic medicine Salacia reticulata. Chem Pharm Bull (Tokyo). 1998. 46:1339–1340.
Article8. Williams JA, Choe YS, Noss MJ, Baumgartner CJ, Mustad VA. Extract of Salacia oblonga lowers acute glycemia in patients with type 2 diabetes. Am J Clin Nutr. 2007. 86:124–130.
Article9. Deguchi Y, Miyazaki K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr Metab (Lond). 2010. 7:9.
Article10. Blonde L. Current antihyperglycemic treatment strategies for patients with type 2 diabetes mellitus. Cleve Clin J Med. 2009. 76:Suppl 5. S4–S11.11. Akase T, Shimada T, Harasawa Y, Ikeya Y, Nagai E, Iizuka S, Nakagami G, Iizaka S, Sanada H, Aburada M. Preventive effects of Salacia reticulata on obesity and metabolic disorders in TSOD mice. Evid Based Complement Alternat Med. 2009. [Epub ahead of print].12. Kishino E, Ito T, Fujita K, Kiuchi Y. A mixture of the Salacia reticulata (Kotala himbutu) aqueous extract and cyclodextrin reduces the accumulation of visceral fat mass in mice and rats with high-fat diet-induced obesity. J Nutr. 2006. 136:433–439.
Article13. Kohchi C, Inagawa H, Nishizawa T, Yamaguchi T, Nagai S, Soma G. Applications of lipopolysaccharide derived from Pantoea agglomerans (IP-PA1) for health care based on macrophage network theory. J Biosci Bioeng. 2006. 102:485–496.
Article14. Tsukioka D, Nishizawa T, Miyase T, Achiwa K, Suda T, Soma G, Mizuno D. Structural characterization of lipid A obtained from Pantoea agglomerans lipopolysaccharide. FEMS Microbiol Lett. 1997. 149:239–244.
Article15. Inagawa H, Nishizawa T, Tsukioka D, Suda T, Chiba Y, Okutomi T, Morikawa A, Soma GI, Mizuno D. Homeostasis as regulated by activated macrophage. II. LPS of plant origin other than wheat flour and their concomitant bacteria. Chem Pharm Bull (Tokyo). 1992. 40:994–997.
Article16. Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, Maisch S, Carr D, Gerlach F, Bufe A, Lauener RP, Schierl R, Renz H, Nowak D, von Mutius E. Allergy and Endotoxin Study Team. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002. 347:869–877.
Article17. Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999. 69:1046S–1051S.
Article18. Uribe A, Alam M, Midtvedt T, Smedfors B, Theodorsson E. Endogenous prostaglandins and microflora modulate DNA synthesis and neuroendocrine peptides in the rat gastrointestinal tract. Scand J Gastroenterol. 1997. 32:691–699.
Article19. Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002. 99:15451–15455.
Article20. Marshall JC. Lipopolysaccharide: an endotoxin or an exogenous hormone? Clin Infect Dis. 2005. 41:Suppl 7. S470–S480.
Article21. Miao Y, Zhou J, Chen C, Shen D, Song W, Feng Y. In vitro adsorption revealing an apparent strong interaction between endophyte Pantoea agglomerans YS19 and host rice. Curr Microbiol. 2008. 57:547–551.
Article22. Asis CA Jr, Adachi K. Isolation of endophytic diazotroph Pantoea agglomerans and nondiazotroph Enterobacter asburiae from sweetpotato stem in Japan. Lett Appl Microbiol. 2004. 38:19–23.
Article23. Pusey PL. Effect of nectar on microbial antagonists evaluated for use in control of fire blight of pome fruits. Phytopathology. 1999. 89:39–46.
Article24. Stockwell VO, Johnson KB, Sugar D, Loper JE. Antibiosis contributes to biological control of fire blight by Pantoea agglomerans strain Eh252 in orchards. Phytopathology. 2002. 92:1202–1209.
Article25. Iguchi M, Inagawa H, Nishizawa T, Okutomi T, Morikawa A, Soma GI, Mizuno D. Homeostasis as regulated by activated macrophage. V. Suppression of diabetes mellitus in non-obese diabetic mice by LPSw (a lipopolysaccharide from wheat flour). Chem Pharm Bull (Tokyo). 1992. 40:1004–1006.
Article26. Okutomi T, Nishizawa T, Inagawa H, Takano T, Morikawa A, Soma G, Mizuno D. Homeostasis as regulated by activated macrophage. VII. Suppression of serum cholesterol level by LPSw (a lipopolysaccharide from wheat flour) in WHHL (Watanabe heritable hyperlipidemic) rabbit. Chem Pharm Bull (Tokyo). 1992. 40:1268–1270.
Article27. Lu Q, Björkhem I, Wretlind B, Diczfalusy U, Henriksson P, Freyschuss A. Effect of ascorbic acid on microcirculation in patients with Type II diabetes: a randomized placebo-controlled cross-over study. Clin Sci (Lond). 2005. 108:507–513.
Article28. Earnest CP, Wood KA, Church TS. Complex multivitamin supplementation improves homocysteine and resistance to LDL-C oxidation. J Am Coll Nutr. 2003. 22:400–407.
Article29. Im R, Mano H, Nakatani S, Shimizu J, Wada M. Safety evaluation of the aqueous extract Kothala himbutu (Salacia reticulata) stem in the hepatic gene expression profile of normal mice using DNA microarrays. Biosci Biotechnol Biochem. 2008. 72:3075–3083.
Article30. Benalla W, Bellahcen S, Bnouham M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr Diabetes Rev. 2010. 6:247–254.
Article31. Geutskens SB, Otonkoski T, Pulkkinen MA, Drexhage HA, Leenen PJ. Macrophages in the murine pancreas and their involvement in fetal endocrine development in vitro. J Leukoc Biol. 2005. 78:845–852.
Article32. Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, Yoon JW. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1999. 189:347–358.
Article33. Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and beta-cell destruction in NOD mice. Diabetes. 1994. 43:667–675.
Article34. Meijer K, de Vries M, Al-Lahham S, Bruinenberg M, Weening D, Dijkstra M, Kloosterhuis N, van der Leij RJ, van der Want H, Kroesen BJ, Vonk R, Rezaee F. Human primary adipocytes exhibit immune cell function: adipocytes prime inflammation independent of macrophages. PLoS One. 2011. 6:e17154.
Article35. Wong FS, Visintin I, Wen L, Flavell RA, Janeway CA Jr. CD8 T cell clones from young nonobese diabetic (NOD) islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. J Exp Med. 1996. 183:67–76.
Article36. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009. 15:914–920.
Article37. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006. 444:860–867.
Article38. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002. 23:549–555.
Article39. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007. 117:175–184.
Article40. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998. 101:890–898.
Article41. Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Di Liberto D, Dieli F, Ghisletti S, Natoli G, De Baetselier P, Mantovani A, Sica A. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009. 106:14978–14983.
Article42. Mabley JG, Pacher P, Southan GJ, Salzman AL, Szabó C. Nicotine reduces the incidence of type I diabetes in mice. J Pharmacol Exp Ther. 2002. 300:876–881.
Article43. Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005. 98:1154–1162.
Article44. Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008. 118:2992–3002.
Article45. Fukushima M, Usami M, Ikeda M, Nakai Y, Taniguchi A, Matsuura T, Suzuki H, Kurose T, Yamada Y, Seino Y. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: a cross-sectional study of Japanese type 2 diabetes. Metabolism. 2004. 53:831–835.
Article46. Xu L, Jiang CQ, Lam TH, Cheng KK, Yue XJ, Lin JM, Zhang WS, Thomas GN. Impact of impaired fasting glucose and impaired glucose tolerance on arterial stiffness in an older Chinese population: the Guangzhou Biobank Cohort Study-CVD. Metabolism. 2010. 59:367–372.
Article47. Lin JD, Wan HL, Li JC, Wu CZ, Kuo SW, Hsieh CH, Lian WC, Lee CH, Kao MT, Pei D. Impaired glucose tolerance and impaired fasting glucose share similar underlying pathophysiologies. Tohoku J Exp Med. 2007. 212:349–357.
Article48. Payne WR, Walsh KJ, Harvey JT, Livy MF, McKenzie KJ, Donaldson A, Atkinson MG, Keogh JB, Moss RS, Dunstan DW, Hubbard WA. Effect of a low-resource-intensive lifestyle modification program incorporating gymnasium-based and home-based resistance training on type 2 diabetes risk in Australian adults. Diabetes Care. 2008. 31:2244–2250.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Pumpkin Water Extract Supplement on Serum Lipid and Fasting Glucose Levels in Female Collegians
- Association of Cardiorespiratory Fitness with Insulin Resistance, Blood Lipids and Bone Mineral Density in Young Female Adults
- New Therapeutic Approaches to the Treatment of Dyslipidemia 2: LDL-C and Lp(a)
- Predictors of Serum Low-Density Lipoprotein Cholesterol Level in Postmenopausal Women
- The Relationship between High Sensitivity C-Reactive Protein and Metabolic Syndrome according to the Fasting Glucose Level at Medical Checkups