Nutr Res Pract.
2011 Oct;5(5):412-420.
Study on the hypochlolesterolemic and antioxidative effects of tyramine derivatives from the root bark of Lycium chenese Miller
- Affiliations
-
- 1Department of Food Science and Nutrition, Catholic University of Daegu, 13-13 Hayang-ro, Hayang-eup, Gyeongsan-si, Gyeongbuk 712-702, Korea. shcho@cu.ac.kr
Abstract
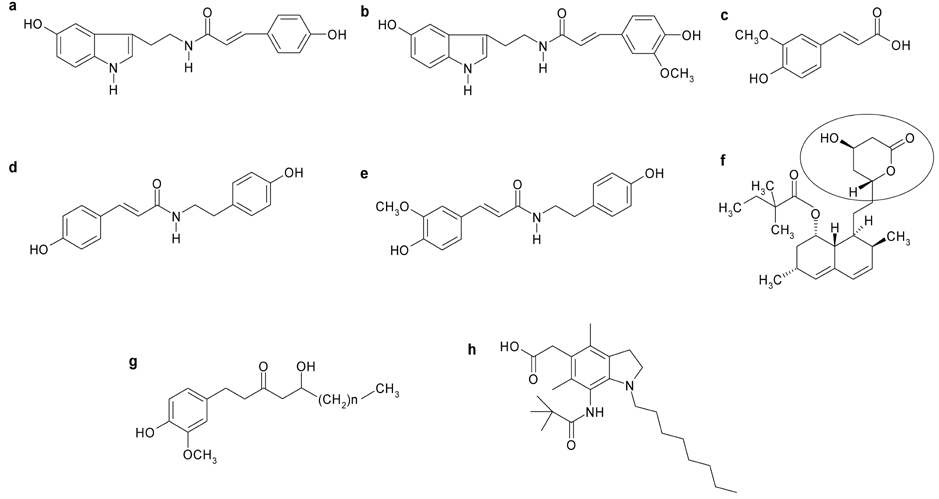
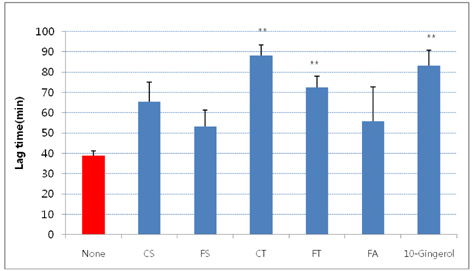
- The aim of the present study was to investigate the hypocholesterolemic effect and potential of tyramine derivatives from Lycii Cortex Radicis (LCR), the root bark of lycium (Lycium chenese Miller) in reducing lipid peroxidation. The activities of enzymes, hepatic 3-hydroxy 3-methylglutaryl (HMG) CoA reductase and acyl-CoA:cholesterol acyltransferase (ACAT) and LDL oxidation were measured in vitro and animal experiments were also performed by feeding LCR extracts to rats. The test compounds employed for in vitro study were trans-N-p-coumaroyltyramine (CT) and trans-N-feruloyltyramine (FT), LCR components, N-(p-coumaroyl)serotonin (CS) and N-feruloylserotonin (FS) from safflower seeds, ferulic acid (FA) and 10-gingerol. It was observed that FT and FS at the concentration of 1.2 mg/mL inhibited liver microsomal HMG CoA reductase activity by ~40%, but no inhibition of activity was seen in the cases of CT, CS, FA and 10-gingerol. Whereas, ACAT activity was inhibited ~50% by FT and CT, 34-43% by FS and CS and ~80% by 10-gingerol at the concentration of 1 mg/mL. A significant delay in LDL oxidation was induced by CT, FT, and 10-gingerol. For the animal experiment, five groups of Sprague-Dawley male rats were fed high fat diets containing no test material (HF-control), 1 and 2% of LCR ethanol extract (LCR1 and LCR2), and 1% of extracts from safflower seed (Saf) and ginger (Gin). The results indicated that total cholesterol level was significantly lower in Saf, LCR2 and Gin groups, and HDL cholesterol level was lower only in Gin group when compared with HF-control group; while there was no difference in the serum triglyceride levels among the five experimental groups. The level of liver cholesterol was significantly lower in LCR1 and LCR2 groups than HF-control. Serum levels of TBARS were significantly lower only in LCR2 group when compared with HF-control group. From the observed results, we concluded that LCR can be utilized as a hypocholesterolemic ingredient in combination with ginger, especially for functional foods.
Keyword
MeSH Terms
-
Acetylmuramyl-Alanyl-Isoglutamine
Animal Experimentation
Animals
Carthamus tinctorius
Catechols
Cholesterol
Cholesterol, HDL
Coumaric Acids
Diet, High-Fat
Ethanol
Fatty Alcohols
Functional Food
Ginger
Humans
Hydroxymethylglutaryl CoA Reductases
Lipid Peroxidation
Liver
Lycium
Male
Oxidoreductases
Polysorbates
Rats
Seeds
Serotonin
Squalene
Thiobarbituric Acid Reactive Substances
Tyramine
Acetylmuramyl-Alanyl-Isoglutamine
Catechols
Cholesterol
Cholesterol, HDL
Coumaric Acids
Ethanol
Fatty Alcohols
Hydroxymethylglutaryl CoA Reductases
Oxidoreductases
Polysorbates
Serotonin
Squalene
Thiobarbituric Acid Reactive Substances
Tyramine
Figure
Reference
-
1. Endo A. Monacolin K, a new hypocholesterolemic agent that specifically inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Antibiot (Tokyo). 1980. 33:334–336.
Article2. Endo A, Komagata D, Shimada H. Monacolin M, a new inhibitor of cholesterol biosynthesis. J Antibiot (Tokyo). 1986. 39:1670–1673.
Article3. Gunde-Cimerman N, Plemenitas A, Cimerman A. A hydroxymethylglutaryl-CoA reductase inhibitor synthesized by yeasts. FEMS Microbiol Lett. 1995. 132:39–43.
Article4. Grundy SM. HMG-CoA reductase inhibitors for treatment of hypercholesterolemia. N Engl J Med. 1988. 319:24–33.
Article5. Qureshi AA, Burger WC, Peterson DM, Elson CE. The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J Biol Chem. 1986. 261:10544–10550.
Article6. Gebhardt R, Beck H. Differential inhibitory effects of garlic-derived organosulfur compounds on cholesterol biosynthesis in primary rat hepatocyte cultures. Lipids. 1996. 31:1269–1276.
Article7. Bok SH, Lee SH, Park YB, Bae KH, Son KH, Jeong TS, Choi MS. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J Nutr. 1999. 129:1182–1185.
Article8. Bok SH, Park SY, Park YB, Lee MK, Jeon SM, Jeong TS, Choi MS. Quercetin dihydrate and gallate supplements lower plasma and hepatic lipids and change activities of hepatic antioxidant enzymes in high cholesterol-fed rats. Int J Vitam Nutr Res. 2002. 72:161–169.
Article9. Ha TY, Cho IJ, Lee SH. Screening of HMG-CoA reductase inhibitory activity of ethanol and methanol extracts from cereals and regumes. Korean J Food Sci Technol. 1998. 30:224–229.10. Cho SH, Park YY, Yoon JY, Choi SW, Ha TY. The effect of polyphenols from safflower seed on HMG-CoA reductase (HMGR) activity, LDL oxidation and apo A1 secretion. Korean J Food Sci Technol. 2006. 38:279–283.11. Pak VV, Koo M, Lee N, Oh SK, Kim MS, Lee JS, Kwon DY. Hypocholesterolemic soybean peptide (IAVP) inhibits HMG-CoA reductase in a competitive manner. Food Sci Biotechnol. 2005. 14:727–731.12. Lee HJ, Choi MS. Measurement of inhibitory activities on 3-hydroxy-3-methylglutaryl CoA reductase and acyl-CoA:cholesterol acyltransferase by various plant extracts in vitro. J Korean Soc Food Sci Nutr. 1999. 28:958–962.13. Kim NJ, Jung EA, Kim DH, Lee SI. Studies on the development of antihyperlipidemic drugs from oriental herbal medicines (I)): antihyperlipidemic activities of oriental herbal medicines. Korean J Pharmacogn. 1999. 30:368–376.14. Kim MK, Kwon BM, Bae KH, Choi DH, Lee HJ, Kim HE, Kim YK. Screening of Acyl-CoA:cholesterol acyltransferase (ACAT) inhibitors from natural sources. Korean J Pharmacogn. 1999. 30:384–396.15. Lee JH, Seo WD, Jeong SH, Jeong TS, Lee WS, Park KH. Human acyl-CoA: cholesterol acyltransferase inhibitory effect of flavonoids from roots of Glycine max (L.) Merr. J Korean Soc Appl Biol Chem. 2006. 49:57–61.16. Kim GS, Jeong TS, Kim YO, Baek NI, Cha SW, Lee JW, Song KS. Human acyl-CoA:cholesterol acyltransferase-inhibiting dammarane triterpenes from Rhus chinensis. J Korean Soc Appl Biol Chem. 2010. 53:417–421.
Article17. Kitayama K, Tanimoto T, Koga T, Terasaka N, Fujioka T, Inaba T. Importance of acyl-coenzyme A:cholesterol acyltransferase 1/2 dual inhibition for anti-atherosclerotic potency of pactimibe. Eur J Pharmacol. 2006. 540:121–130.
Article18. Terasaka N, Miyazaki A, Kasanuki N, Ito K, Ubukata N, Koieyama T, Kitayama K, Tanimoto T, Maeda N, Inaba T. ACAT inhibitor pactimibe sulfate (CS-505) reduces and stabilizes atherosclerotic lesions by cholesterol-lowering and direct effects in apolipoprotein E-deficient mice. Atherosclerosis. 2007. 190:239–247.
Article19. Ikenoya M, Yoshinaka Y, Kobayashi H, Kawamine K, Shibuya K, Sato F, Sawanobori K, Watanabe T, Miyazaki A. A selective ACAT-1 inhibitor, K-604, suppresses fatty streak lesions in fat-fed hamsters without affecting plasma cholesterol levels. Atherosclerosis. 2007. 191:290–297.
Article20. Nissen SE, Tuzcu EM, Brewer HB, Sipahi I, Nicholls SJ, Ganz P, Schoenhagen P, Waters DD, Pepine CJ, Crowe TD, Davidson MH, Deanfield JE, Wisniewski LM, Hanyok JJ, Kassalow LM. ACAT Intravascular Atherosclerosis Treatment Evaluation (ACTIVATE) Investigators. Effect of ACAT inhibition on the progression of coronary atherosclerosis. N Engl J Med. 2006. 354:1253–1263.
Article21. Witzum JL. Leaf A, Weber PC, editors. The role of monocytes and oxidized LDL in atherosclerosis. Atherosclerosis Reviews. 1990. Vol. 21. New York: Raven Press;59–69.22. Dieber-Rotheneder M, Puhl H, Waeg G, Striegl G, Esterbauer H. Effect of oral supplementation with D-α-tocopherol on the vitamin E content of human low density lipoproteins and resistance to oxidation. J Lipid Res. 1991. 32:1325–1332.
Article23. Meng QH, Lewis P, Wähälä K, Adlercreutz H, Tikkanen MJ. Incorporation of esterified soybean isoflavones with antioxidant activity into low density lipoprotein. Biochim Biophys Acta. 1999. 1438:369–376.
Article24. Gao D, Li Q, Liu Z, Li Y, Liu Z, Fan Y, Li K, Han Z, Li J. Hypoglycemic effects and mechanisms of action of Cortex Lycii Radicis on alloxan-induced diabetic mice. Yakugaku Zasshi. 2007. 127:1715–1721.
Article25. Ahn BY, Gwak JS, Ryu SH, Moon GS, Choi DS, Park SH, Han JH. Protective effect of water extract of Lycii Cordex Radicis on lipid peroxidation of rat skin exposed to ultraviolet B radiation. Agric Chem Biotechnol. 2002. 45:218–222.26. Cho YJ, Kim SH. Protective effect of EA fraction of Lycii Cortex Radix on the hepatic damage in mice induced by CCl4. Korean J Orient Med Pathol. 1997. 11:63–71.27. Lee DG, Park Y, Kim MR, Jung HJ, Seu YB, Hahm KS, Woo ER. Anti-fungal effects of phenolic amides isolated from the root bark of Lycium chenense. Biotechnol Lett. 2004. 26:1125–1130.
Article28. Kang GH, Chang EJ, Choi SW. Antioxidative activity of phenolic compounds in roasted safflower (Carthamus tinctorius L.) seeds. J Korean Soc Food Sci Nutr. 1999. 4:221–225.29. Rajan P, Vedernikova I, Cos P, Berghe DV, Augustyns K, Haemers A. Synthesis and evaluation of caffeic acid amides as antioxidants. Bioorg Med Chem Lett. 2001. 11:215–217.
Article30. Kim EO, Son WR, Kwon SO, Choi SW. Antilipidemic activity of phenolic compounds isolated from plant sources. Abstracts from Annual Meeting of Korean Society of Food Sci & Technology. 2010. Incheon. 11–75.31. Kleinsek DA, Dugan RE, Baker TA, Porter JW. Lowenstein JM, editor. 3-Hydroxy-3-methylglutaryl coenzyme A reductase from rat liver. Methods in Enzymology. 1981. Vol. 71. New York: Academic Press;462–479.32. Hirano R, Kondo K, Iwamoto T, Igarashi O, Itakura H. Effects of antioxidants on the oxidative susceptibility of low-density lipoprotein. J Nutr Sci Vitaminol (Tokyo). 1997. 43:435–444.
Article33. Woo MN, Bok SH, Choi MS. Hypolipidemic and body fat-lowering effects of Fatclean in rats fed a high-fat diet. Food Chem Toxicol. 2009. 47:2076–2082.
Article34. Yang SY, Hong S, Sung MK, Kang MH, Kim MK. Effect of lecithin intake on lipid metabolism and antioxidative capacity in rats fed high fat diet. Korean J Nutr. 2007. 40:312–319.35. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993. 123:1939–1951.
Article36. Cho SH, Lee HR, Kim TH, Choi SW, Lee WJ, Choi Y. Effects of defatted safflower seed extract and phenolic compounds in diet on plasma and liver lipid in ovariectomized rats fed high-cholesterol diets. J Nutr Sci Vitaminol (Tokyo). 2004. 50:32–37.
Article37. Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976. 15:212–216.
Article38. Chow LW, Wang SJ, Duh PD. Antibacterial activity of burdock. Food Sci. 1997. 24:195–202.39. Roberfroid MB, Delzenne NM. Dietary fructans. Annu Rev Nutr. 1998. 18:117–143.
Article40. Sung HY, Jeoung HJ, Choi YS, Cho SH, Yun JW. Effects of chicory inulin and oligosaccharides on lipid metabolism in rats fed a high-cholesterol diet. J Korean Soc Food Sci Nutr. 2004. 33:305–310.
Article41. Her BY, Sung HY, Choi YS. Oligosaccharide-supplemented soy ice cream for diabetic patients: quality characteristics and effects on blood sugar and lipids in streptozotocin-induced diabetic rats. Korean J Nutr. 2005. 38:663–671.42. Lee YS, Kang EY, Park MN, Choi YY, Jeon JW, Yun SS. Effects of sn-2 palmitic acid-fortified vegetable oil and fructooligosaccharide on calcium metabolism in growing rats fed casein based diet. Nutr Res Pract. 2008. 2:3–7.
Article43. Jung UJ, Baek NI, Chung HG, Jeong TS, Lee KT, Lee MK, Choi MS. Antilipogenic and hypolipidemic effects of ethanol extracts from two variants of Artemisia princeps Pampanini in obese diabetic mice. J Med Food. 2009. 12:1238–1244.
Article44. Kwon EY, Do GM, Cho YY, Park YB, Jeon SM, Choi MS. Anti-atherogenic property of ferulic acid in apolipoprotein E-deficient mice fed Western diet: comparison with clofibrate. Food Chem Toxicol. 2010. 48:2298–2303.
Article45. Sri Balasubashini M, Rukkumani R, Menon VP. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003. 40:118–122.
Article46. Oyagbemi AA, Saba AB, Azeez OI. Molecular targets of [6]-gingerol: Its potential roles in cancer chemoprevention. Biofactors. 2010. 36:169–178.
Article47. Nigam N, George J, Srivastava S, Roy P, Bhui K, Singh M, Shukla Y. Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B[a]P-induced mouse skin tumorigenesis. Cancer Chemother Pharmacol. 2010. 65:687–696.
Article48. Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010. 127:515–520.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of the purified extracts from Lycii Cortex Radicis and ginger on lipid statusand serum cytokine levels in rats fed high fat diet
- Effect of toloxatone on the pressor effect of tyramine in rat: Comparison with monoamine oxidase inhibition by iproniazid
- The Effects of the Ulmus Root-bark Dressing in Tissue Regeneration of Induced Pressure Ulcers in Rats
- The Infuluences of Sympathomimetic Amines on Melanophores of the Frog Skin
- Root coverage using subepithelial connective tissue graft