Nutr Res Pract.
2011 Oct;5(5):396-403.
Analysis of ceramide metabolites in differentiating epidermal keratinocytes treated with calcium or vitamin C
- Affiliations
-
- 1Department of Medical Nutrition, Graduate School of East-West Medical Science, Kyung Hee University, Seocheon-dong, Giheung-gu, Yongin-si, Gyeonggi 446-701, Korea. choyunhi@khu.ac.kr
Abstract
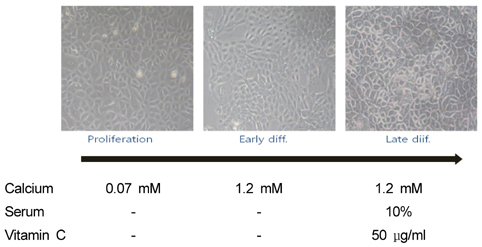
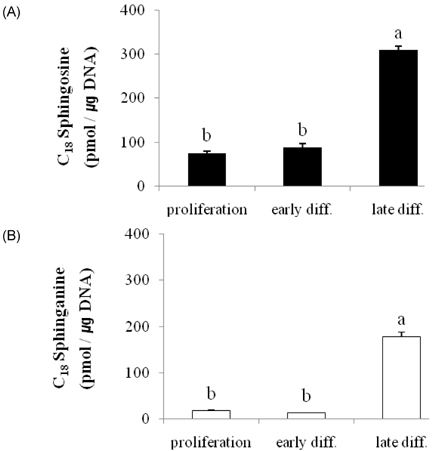
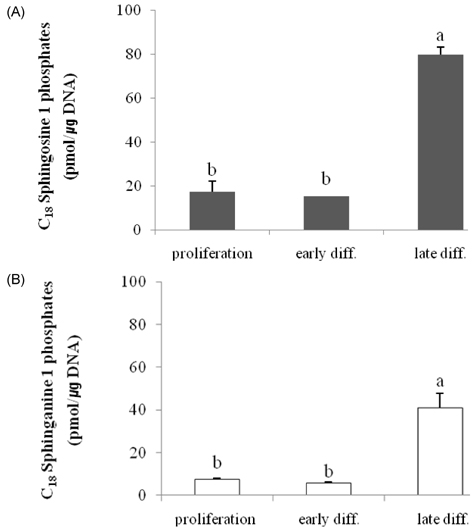
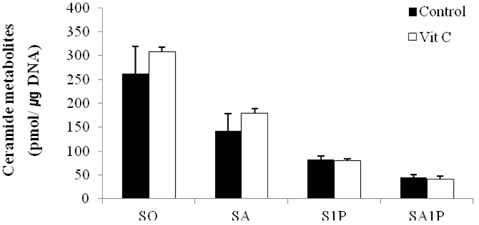
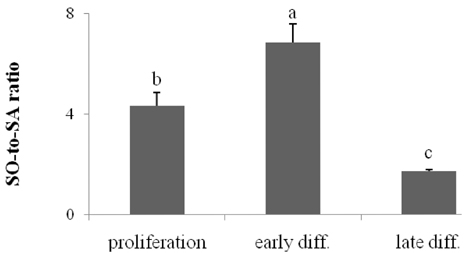
- Ceramides (Cer) comprise the major constituent of sphingolipids in the epidermis and are known to play diverse roles in the outermost layers of the skin including water retention and provision of a physical barrier. In addition, they can be hydrolyzed into free sphingoid bases such as C18 sphingosine (SO) and C18 sphinganine (SA) or can be further metabolized to C18 So-1-phosphate (S1P) and C18 Sa-1-phosphate (Sa1P) in keratinocytes. The significance of ceramide metabolites emerged from studies reporting altered levels of SO and SA in skin disorders and the role of S1P and Sa1P as signaling lipids. However, the overall metabolism of sphingoid bases and their phosphates during keratinocyte differentiation remains not fully understood. Therefore, in this study, we analyzed these Cer metabolites in the process of keratinocyte differentiation. Three distinct keratinocyte differentiation stages were prepared using 0.07 mM calcium (Ca2+) (proliferation stage), 1.2 mM Ca2+ (early differentiation stage) in serum-free medium, or serum-containing medium with vitamin C (50 microL/mL) (late differentiation stage). Serum-containing medium was also used to determine whether vitamin C increases the concentrations of sphingoid bases and their phosphates. The production of sphingoid bases and their phosphates after hydrolysis by alkaline phosphatase was determined using high-performance liquid chromatography. Compared to cells treated with 0.07 mM Ca2+, levels of SO, SA, S1P, and SA1P were not altered after treatment with 1.2 mM Ca2+. However, in keratinocytes cultured in serum-containing medium with vitamin C, levels of SO, SA, S1P, and SA1P were dramatically higher than those in 0.07- and 1.2-mM Ca2+-treated cells; however, compared to serum-containing medium alone, vitamin C did not significantly enhance their production. Taken together, we demonstrate that late differentiation induced by vitamin C and serum was accompanied by dramatic increases in the concentration of sphingoid bases and their phosphates, although vitamin C alone had no effect on their production.
Keyword
MeSH Terms
Figure
Reference
-
1. Houben E, De Paepe K, Rogiers V. A keratinocyte's course of life. Skin Pharmacol Physiol. 2007. 20:122–132.
Article2. Downing DT, Stewart ME, Wertz PW, Colton SW, Abraham W, Strauss JS. Skin lipids: an update. J Invest Dermatol. 1987. 88:2s–6s.
Article3. Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv Lipid Res. 1991. 24:1–26.
Article4. Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980. 19:245–254.
Article5. Ponec M, Weerheim A, Kempenaar J, Mulder A, Gooris GS, Bouwstra J, Mommaas AM. The formation of competent barrier lipids in reconstructed human epidermis requires the presence of vitamin C. J Invest Dermatol. 1997. 109:348–355.
Article6. Uchida Y, Behne M, Quiec D, Elias PM, Holleran WM. Vitamin C stimulates sphingolipid production and markers of barrier formation in submerged human keratinocyte cultures. J Invest Dermatol. 2001. 117:1307–1313.
Article7. Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985. 84:508–512.
Article8. Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006. 580:5456–5466.
Article9. Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 2009. 91:784–790.
Article10. Geilen CC, Barz S, Bektas M. Sphingolipid signaling in epidermal homeostasis. Current knowledge and new therapeutic approaches in dermatology. Skin Pharmacol Appl Skin Physiol. 2001. 14:261–271.11. Wertz PW, Downing DT. Free sphingosine in human epidermis. J Invest Dermatol. 1990. 94:159–161.
Article12. Wertz PW, Downing DT. Free sphingosines in porcine epidermis. Biochim Biophys Acta. 1989. 1002:213–217.
Article13. Wakita H, Nishimura K, Takigawa M. Composition of free long-chain (sphingoid) bases in stratum corneum of normal and pathologic human skin conditions. J Invest Dermatol. 1992. 99:617–622.
Article14. Bibel DJ, Aly R, Shinefield HR. Antimicrobial activity of sphingosines. J Invest Dermatol. 1992. 98:269–273.
Article15. Arikawa J, Ishibashi M, Kawashima M, Takagi Y, Ichikawa Y, Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J Invest Dermatol. 2002. 119:433–439.
Article16. Gupta AK, Fisher GJ, Elder JT, Nickoloff BJ, Voorhees JJ. Sphingosine inhibits phorbol ester-induced inflammation, ornithine decarboxylase activity, and activation of protein kinase C in mouse skin. J Invest Dermatol. 1988. 91:486–491.
Article17. Kawanabe T, Kawakami T, Yatomi Y, Shimada S, Soma Y. Sphingosine 1-phosphate accelerates wound healing in diabetic mice. J Dermatol Sci. 2007. 48:53–60.
Article18. Vogler R, Sauer B, Kim DS, Schäfer-Korting M, Kleuser B. Sphingosine-1-phosphate and its potentially paradoxical effects on critical parameters of cutaneous wound healing. J Invest Dermatol. 2003. 120:693–700.
Article19. Houben E, Holleran WM, Yaginuma T, Mao C, Obeid LM, Rogiers V, Takagi Y, Elias PM, Uchida Y. Differentiation-associated expression of ceramidase isoforms in cultured keratinocytes and epidermis. J Lipid Res. 2006. 47:1063–1070.
Article20. Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980. 102:344–352.
Article21. Ruwisch L, Schäfer-Korting M, Kleuser B. An improved high-performance liquid chromatographic method for the determination of sphingosine-1-phosphate in complex biological materials. Naunyn Schmiedebergs Arch Pharmacol. 2001. 363:358–363.
Article22. Min JK, Yoo HS, Lee EY, Lee WJ, Lee YM. Simultaneous quantitative analysis of sphingoid base 1-phosphates in biological samples by o-phthalaldehyde precolumn derivatization after dephosphorylation with alkaline phosphatase. Anal Biochem. 2002. 303:167–175.
Article23. Yoon HT, Yoo HS, Shin BK, Lee WJ, Kim HM, Hong SP, Moon DC, Lee YM. Improved fluorescent determination method of cellular sphingoid bases in high-performance liquid chromatography. Arch Pharm Res. 1999. 22:294–299.
Article24. Pillai S, Bikle DD, Hincenbergs M, Elias PM. Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J Cell Physiol. 1988. 134:229–237.
Article25. Herzinger T, Kleuser B, Schäfer-Korting M, Korting HC. Sphingosine-1-phosphate signaling and the skin. Am J Clin Dermatol. 2007. 8:329–336.
Article26. Youm JK, Jo H, Hong JH, Shin DM, Kwon MJ, Jeong SK, Park BD, Choi EH, Lee SH. K6PC-5, a sphingosine kinase activator, induces anti-aging effects in intrinsically aged skin through intracellular Ca2+ signaling. J Dermatol Sci. 2008. 51:89–102.
Article27. Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schäfer-Korting M, Kleuser B. 1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J Invest Dermatol. 2001. 117:1241–1249.
Article28. Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta. 2008. 1781:424–434.
Article29. Sun W, Xu R, Hu W, Jin J, Crellin HA, Bielawski J, Szulc ZM, Thiers BH, Obeid LM, Mao C. Upregulation of the human alkaline ceramidase 1 and acid ceramidase mediates calcium-induced differentiation of epidermal keratinocytes. J Invest Dermatol. 2008. 128:389–397.
Article30. Coderch L, López O, de la Maza A, Parra JL. Ceramides and skin function. Am J Clin Dermatol. 2003. 4:107–129.
Article31. Castiel-Higounenc I, Chopart M, Ferraris C. Stratum corneum lipids: specificity, role, deficiencies and modulation. Eur J Dermatol. 2004. 11:401–406.
Article32. Sando GN, Howard EJ, Madison KC. Induction of ceramide glucosyltransferase activity in cultured human keratinocytes. Correlation with culture differentiation. J Biol Chem. 1996. 271:22044–22051.
Article33. Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J Dermatol Sci. 2008. 51:77–87.
Article34. Behne M, Uchida Y, Seki T, de Montellano PO, Elias PM, Holleran WM. Omega-hydroxyceramides are required for corneocyte lipid envelope (CLE) formation and normal epidermal permeability barrier function. J Invest Dermatol. 2000. 114:185–192.
Article35. Ternes P, Franke S, Zähringer U, Sperling P, Heinz E. Identification and characterization of a sphingolipid delta 4-desaturase family. J Biol Chem. 2002. 277:25512–25518.
Article36. Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007. 5:167–179.
Article37. Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R. Ceramide composition of the psoriatic scale. Biochim Biophys Acta. 1993. 1182:147–151.
Article38. Pitson SM, D'andrea RJ, Vandeleur L, Moretti PA, Xia P, Gamble JR, Vadas MA, Wattenberg BW. Human sphingosine kinase: purification, molecular cloning and characterization of the native and recombinant enzymes. Biochem J. 2000. 350:429–441.
Article39. Olivera A, Kohama T, Tu Z, Milstien S, Spiegel S. Purification and characterization of rat kidney sphingosine kinase. J Biol Chem. 1998. 273:12576–12583.
Article40. Meyer zu Heringdorf D, Lass H, Alemany R, Laser KT, Neumann E, Zhang C, Schmidt M, Rauen U, Jakobs KH, van Koppen CJ. Sphingosine kinase-mediated Ca2+ signalling by G-protein-coupled receptors. EMBO J. 1998. 17:2830–2837.41. Hong JH, Youm JK, Kwon MJ, Park BD, Lee YM, Lee SI, Shin DM, Lee SH. K6PC-5, a direct activator of sphingosine kinase 1, promotes epidermal differentiation through intracellular Ca2+ signaling. J Invest Dermatol. 2008. 128:2166–2178.
Article42. Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, Beech DJ. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006. 98:1381–1389.
Article43. Bornfeldt KE, Graves LM, Raines EW, Igarashi Y, Wayman G, Yamamura S, Yatomi Y, Sidhu JS, Krebs EG, Hakomori S, Ross R. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cells: spatial and temporal modulation of PDGF chemotactic signal transduction. J Cell Biol. 1995. 130:193–206.
Article44. Sauer B, Vogler R, von Wenckstern H, Fujii M, Anzano MB, Glick AB, Schäfer-Korting M, Roberts AB, Kleuser B. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J Biol Chem. 2004. 279:38471–38479.
Article45. Kim J, Kim Y, Yun H, Park H, Kim SY, Lee KG, Han SM, Cho Y. Royal jelly enhances migration of human dermal fibroblasts and alters the levels of cholesterol and sphinganine in an in vitro wound healing model. Nutr Res Pract. 2010. 4:362–368.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vitamin C Stimulates Epidermal Ceramide Production by Regulating Its Metabolic Enzymes
- Vitamin D and Immune Responses
- Skin Barrier and Calcium
- Effects of calcipotriol(MC 903), a novel synthetic derivative of vitamin D3 on the growth of cultured human keratinocytes and melanocytes
- Associations among plasma vitamin C, epidermal ceramide and clinical severity of atopic dermatitis