Nutr Res Pract.
2011 Jun;5(3):185-191.
Chestnut extract induces apoptosis in AGS human gastric cancer cells
- Affiliations
-
- 1Department of Food and Nutrition, Kookmin University, Seoul 136-702, Korea.
- 2Center for Efficacy Assessment and Development of Functional Foods and Drugs, Hallym University, Chuncheon, Gangwon 200-702, Korea.
- 3Department of Foodservice Management and Nutrition, Kongju National University, 182 Shinkwan-dong, Gongju, Chungnam 314-701, Korea. shkim@kongju.ac.kr
Abstract
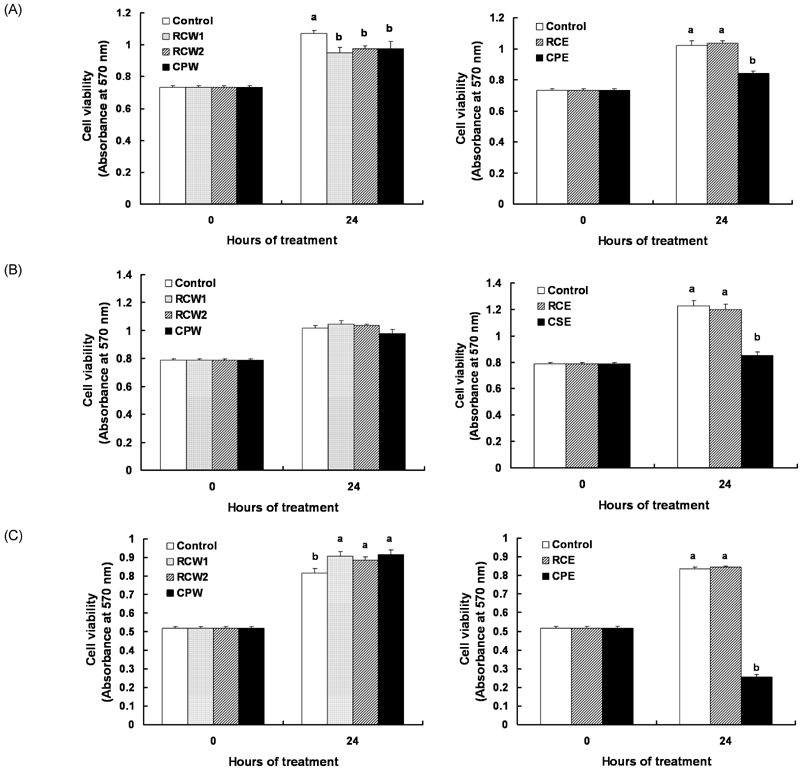
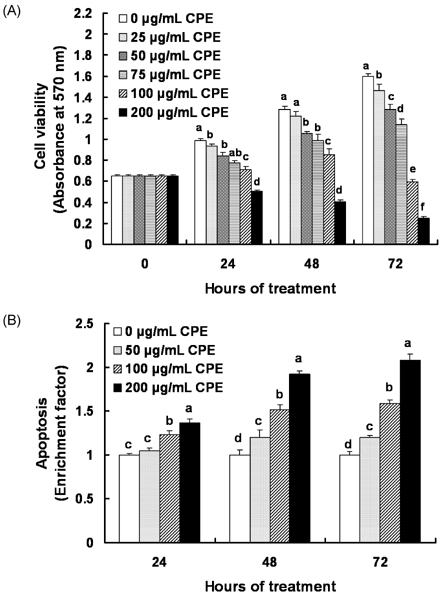
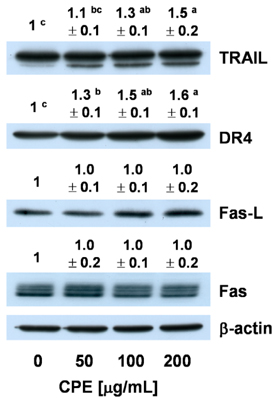
- In Korea, chestnut production is increasing each year, but consumption is far below production. We investigated the effect of chestnut extracts on antioxidant activity and anticancer effects. Ethanol extracts of raw chestnut (RCE) or chestnut powder (CPE) had dose-dependent superoxide scavenging activity. Viable numbers of MDA-MD-231 human breast cancer cells, DU145 human prostate cancer cells, and AGS human gastric cancer cells decreased by 18, 31, and 69%, respectively, following treatment with 200 microg/mL CPE for 24 hr. CPE at various concentrations (0-200 microg/mL) markedly decreased AGS cell viability and increased apoptotic cell death dose and time dependently. CPE increased the levels of cleaved caspase-8, -7, -3, and poly (ADP-ribose) polymerase in a dose-dependent manner but not cleaved caspase-9. CPE exerted no effects on Bcl-2 and Bax levels. The level of X-linked inhibitor of apoptosis protein decreased within a narrow range following CPE treatment. The levels of Trail, DR4, and Fas-L increased dose-dependently in CPE-treated AGS cells. These results show that CPE decreases growth and induces apoptosis in AGS gastric cancer cells and that activation of the death receptor pathway contributes to CPE-induced apoptosis in AGS cells. In conclusion, CPE had more of an effect on gastric cancer cells than breast or prostate cancer cells, suggesting that chestnuts would have a positive effect against gastric cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Paillet FL. Chestnut: history and ecology of a transformed species. J Biogeogr. 2002. 29:1517–1530.
Article2. Kim JS, Jung BH, Joo RW, Choi SI. Marketing of chestnut and economic analysis of chestnut cultivation. Korean J For Econ. 2004. 12:12–21.3. Heo J. Doguibogam. 2001. Seoul: Hanmibook press;1153.4. Vekiari SA, Gordon MH, García-Macías P, Labrinea H. Extraction and determination of ellagic acid content in chestnut bark and fruit. Food Chem. 2008. 110:1007–1011.
Article5. Barreira JCM, Ferreira ICFR, Oliveira MBPP, Pereira JA. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008. 107:1106–1113.
Article6. Kim EJ, Kang IJ, Cho HJ, Kim WK, Ha YL, Park JH. Conjugated linoleic acid downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. J Nutr. 2003. 133:2675–2681.
Article7. Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003. 284:G996–G1005.8. Na MH, Seo EY, Kim WK. Effects of α-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells. Nutr Res Pract. 2009. 3:265–271.
Article9. Chen C, Kong AN. Dietary cancer-chemopreventive compounds: from signaling and gene expression to pharmacological effects. Trends Pharmacol Sci. 2005. 26:318–326.
Article10. Jeon BG, Jeong HW, Lee JR, Ji JM. The development of functional beverage from the inner skin of the chestnut Castanea crenata (II) - Physiological effects of chestnut inner skin tea, brown rice-green tea and Cassia tora tea in mouse and rat. Korean J Food Nutr. 2000. 13:411–418.11. Lorenzo HK, Susin SA. Therapeutic potential of AIF-mediated caspase-independent programmed cell death. Drug Resist Updat. 2007. 10:235–255.
Article12. Oliver L, Vallette FM. The role of caspases in cell death and differentiation. Drug Resist Updat. 2005. 8:163–170.
Article13. Iannolo G, Conticello C, Memeo L, De Maria R. Apoptosis in normal and cancer stem cells. Crit Rev Oncol Hematol. 2008. 66:42–51.
Article14. Qiao L, Wong BC. Targeting apoptosis as an approach for gastrointestinal cancer therapy. Drug Resist Updat. 2009. 12:55–64.
Article15. Thorburn A, Behbakht K, Ford H. TRAIL receptor-targeted therapeutics: resistance mechanisms and strategies to avoid them. Drug Resist Updat. 2008. 11:17–24.
Article16. Sträter J, Möller P. CD95 (Fas/APO-1)/CD95L in the gastrointestinal tract: fictions and facts. Virchows Arch. 2003. 442:218–225.
Article17. Nagashima H, Mori M, Sadanaga N, Mashino K, Yoshikawa Y, Sugimachi K. Expression of Fas ligand in gastric carcinoma relates to lymph node metastasis. Int J Oncol. 2001. 18:1157–1162.
Article18. Lim SC. Fas-related apoptosis in gastric adenocarcinoma. Oncol Rep. 2003. 10:57–63.
Article19. Korkolopoulou P, Saetta AA, Levidou G, Gigelou F, Lazaris A, Thymara I, Scliri M, Bousboukea K, Michalopoulos NV, Apostolikas N, Konstantinidou A, Tzivras M, Patsouris E. c-FLIP expression in colorectal carcinomas: association with Fas/FasL expression and prognostic implications. Histopathology. 2007. 51:150–156.
Article20. Mahalingam D, Szegezdi E, Keane M, de Jong S, Samali A. TRAIL receptor signalling and modulation: are we on the right TRAIL? Cancer Treat Rev. 2009. 35:280–288.
Article21. Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999. 15:269–290.
Article22. Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998. 281:1305–1308.
Article23. Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998. 17:3261–3270.
Article24. Verhagen AM, Silke J, Ekert PG, Pakusch M, Kaufmann H, Connolly LM, Day CL, Tikoo A, Burke R, Wrobel C, Moritz RL, Simpson RJ, Vaux DL. HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J Biol Chem. 2002. 277:445–454.
Article25. LaCasse EC, Cherton-Horvat GG, Hewitt KE, Jerome LJ, Morris SJ, Kandimalla ER, Yu D, Wang H, Wang W, Zhang R, Agrawal S, Gillard JW, Durkin JP. Preclinical characterization of AEG35156/GEM 640, a second-generation antisense oligonucleotide targeting X-linked inhibitor of apoptosis. Clin Cancer Res. 2006. 12:5231–5241.
Article26. Tong QS, Zheng LD, Wang L, Zeng FQ, Chen FM, Dong JH, Lu GC. Down-regulation of XIAP expression induces apoptosis and enhances chemotherapeutic sensitivity in human gastric cancer cells. Cancer Gene Ther. 2005. 12:509–514.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of CD40 in Gastric Cancer and Its Effect on the Apoptosis of Gastric Cancer Cells
- Econazole Induces p53-Dependent Apoptosis and Decreases Metastasis Ability in Gastric Cancer Cells
- Apoptosis Induction of Stomach Cancer Cell by TNF alpha and TGFbeta
- Rhus verniciflua Stokes extract suppresses migration and invasion in human gastric adenocarcinoma AGS cells
- Kahweol from Coffee Induces Apoptosis by Upregulating Activating Transcription Factor 3 in Human Colorectal Cancer Cells