Nutr Res Pract.
2010 Oct;4(5):369-374.
Effect of dietary supplementation of grape skin and seeds on liver fibrosis induced by dimethylnitrosamine in rats
- Affiliations
-
- 1Department of Food and Nutrition, Silla University, Busan 617-736, Korea.
- 2College of Pharmacy, Pusan National University, Jangjeon-dong, Geumjeong-gu, Busan 609-735, Korea. mjo@pusan.ac.kr
Abstract
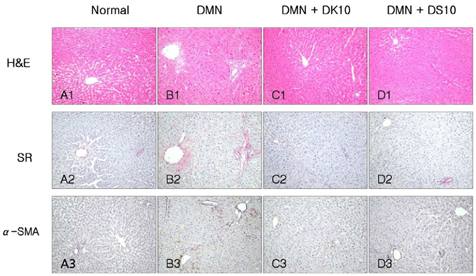
- Grape is one of the most popular and widely cultivated fruits in the world. Although grape skin and seeds are waste product of the winery and grape juice industry, these wastes contain large amounts of phytochemicals such as flavonoids, phenolic acids, and anthocyanidins, which play an important role as chemopreventive and anticancer agents. We evaluated efficacies of grape skin and seeds on hepatic injury induced by dimethylnitrosamine (DMN) in rats. Treatment with DMN significantly increased levels of serum alanine transaminase, aspartate transaminase, alkaline phosphatase, and bilirubin. Diet supplementation with grape skin or seeds (10% daily for 4 weeks) prevented these elevations. The grape skin and seeds also restored serum albumin and total protein levels, and reduced the hepatic level of hydroxyproline and malondialdehyde. Furthermore, grape skin and seeds reduced DMN-induced collagen accumulation, as estimated by histological analysis of liver tissue stained with Sirius red. Grape skin and seeds also reduced hepatic stellate cell activation, as assessed by alpha-smooth muscle actin staining. In conclusion, grape skin and seeds exhibited in vivo hepatoprotective and antifibrogenic effects against DMN-induced liver injury, suggesting that grape skin and seeds may be useful in preventing the development of hepatic fibrosis.
Keyword
MeSH Terms
-
Actins
Alanine Transaminase
Alkaline Phosphatase
Animals
Anthocyanins
Antineoplastic Agents
Aspartate Aminotransferases
Bilirubin
Collagen
Diet
Dietary Supplements
Dimethylnitrosamine
Fibrosis
Flavonoids
Fruit
Hepatic Stellate Cells
Hydroxyproline
Liver
Liver Cirrhosis
Malondialdehyde
Muscles
Phenol
Rats
Seeds
Serum Albumin
Skin
Vitis
Waste Products
Actins
Alanine Transaminase
Alkaline Phosphatase
Anthocyanins
Antineoplastic Agents
Aspartate Aminotransferases
Bilirubin
Collagen
Dimethylnitrosamine
Flavonoids
Hydroxyproline
Malondialdehyde
Phenol
Serum Albumin
Waste Products
Figure
Reference
-
1. Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008. 134:1655–1669.
Article2. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008. 88:125–172.
Article3. Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007. 131:1728–1734.
Article4. Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J Gastroenterol Hepatol. 1999. 14:618–633.
Article5. Nassiri-Asl M, Hosseinzadeh H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive compounds. Phytother Res. 2009. 23:1197–1204.
Article6. Lazzè MC, Pizzala R, Gutiérrez Pecharromán FJ, Gatòn Garnica P, Antolín Rodríguez JM, Fabris N, Bianchi L. Grape waste extract obtained by supercritical fluid extraction contains bioactive antioxidant molecules and induces antiproliferative effects in human colon adenocarcinoma cells. J Med Food. 2009. 12:561–568.
Article7. Haggerty HG, Holsapple MP. Role of metabolism in dimethylnitrosamine-induced immunosuppression: a review. Toxicology. 1990. 63:1–23.
Article8. Terracini B, Magee PN, Barnes JM. Hepatic pathology in rats on low dietary levels of dimethylnitrosamine. Br J Cancer. 1967. 21:559–565.
Article9. Weng HL, Cai WM, Liu RH. Animal experiment and clinical study of effect of gamma-interferon on hepatic fibrosis. World J Gastroenterol. 2001. 7:42–48.
Article10. Jezequel AM, Mancini R, Rinaldesi ML, Ballardini G, Fallani M, Bianchi F, Orlandi F. Dimethylnitrosamine-induced cirrhosis. Evidence for an immunological mechanism. J Hepatol. 1989. 8:42–52.11. Lee ES, Lee HE, Shin JY, Yoon S, Moon JO. The flavonoid quercetin inhibits dimethylnitrosamine-induced liver damage in rats. J Pharm Pharmacol. 2003. 55:1169–1174.
Article12. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951. 193:265–275.
Article13. Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979. 11:447–455.
Article14. Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961. 93:440–447.
Article15. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978. 52:302–310.16. Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol. 2006. 12:7413–7420.
Article17. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005. 115:209–218.
Article18. Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000. 275:2247–2250.
Article19. Zhu W, Fung PC. The roles played by crucial free radicals like lipid free radicals, nitric oxide, and enzymes NOS and NADPH in CCl(4)-induced acute liver injury of mice. Free Radic Biol Med. 2000. 29:870–880.
Article20. Vendemiale G, Grattagliano I, Caruso ML, Serviddio G, Valentini AM, Pirrelli M, Altomare E. Increased oxidative stress in dimethylnitrosamine-induced liver fibrosis in the rat: effect of N-acetylcysteine and interferon-alpha. Toxicol Appl Pharmacol. 2001. 175:130–139.
Article21. Tsukamoto H, Rippe R, Niemela O, Lin M. Role of oxidative stress in activation of Kupffer cell and Ito cells in liver fibrogenesis. J Gastroenterol Hepatol. 1995. 10:S50–S53.22. Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentillini A, Gentillini P, Dianzani MU. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun. 1993. 194:1044–1050.
Article23. Falchi M, Bertelli A, Lo Scalzo R, Morassut M, Morelli R, Das S, Cui J, Das DK. Comparison of cardioprotective abilities between the flesh and skin of grapes. J Agric Food Chem. 2006. 54:6613–6622.
Article24. Yadav M, Jain S, Bhardwaj A, Nagpal R, Puniya M, Tomar R, Singh V, Parkash O, Prasad GB, Marotta F, Yadav H. Biological and medicinal properties of grapes and their bioactive constituents: an update. J Med Food. 2009. 12:473–484.
Article25. Chávez E, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. Resveratrol prevents fibrosis, NF-kappaB activation and TGF-beta increases induced by chronic CCl4 treatment in rats. J Appl Toxicol. 2008. 28:35–43.
Article26. Lee ES, Shin MO, Yoon S, Moon JO. Resveratrol inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Arch Pharm Res. 2010. 33:925–932.
Article27. Dulundu E, Ozel Y, Topaloglu U, Toklu H, Ercan F, Gedik N, Sener G. Grape seed extract reduces oxidative stress and fibrosis in experimental biliary obstruction. J Gastroenterol Hepatol. 2007. 22:885–892.
Article28. Shin MO, Yoon S, Moon JO. The proanthocyanidins inhibit dimethylnitrosamine-induced liver damage in rats. Arch Pharm Res. 2010. 33:167–173.
Article29. Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutr Res. 2008. 28:729–737.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Suppression of oxidative stress by grape seed supplementation in rats
- Effect of Dietary Grape Pomace on Lipid Oxidation and Related Enzyme Activities in Rats Fed High Fat Diet
- Grape skin improves antioxidant capacity in rats fed a high fat diet
- Effects of grape pomace on the antioxidant defense system in diet-induced hypercholesterolemic rabbits
- The Effect of Isoflavone and/or Grape Seed Oil Supplementation on Blood Lipid Profiles and Bone Strength in Ovariectomized Female Rats