Nutr Res Pract.
2010 Oct;4(5):356-361.
Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells
- Affiliations
-
- 1Institute of Marine Biotechnology, Andong National University, 388 Songchun-dong, Andong, Kyungpook 760-749, Korea. bp7122@nate.com
- 2Department of Food Science and Nutrition, Andong National University, Kyungpook 760-749, Korea.
- 3Department of Food Science and Biotechnology, Andong National University, Kyungpook 760-749, Korea.
- 4Department of Oral Pathology, School of Dentistry, Kyungpook NationalUniversity, Daegu 700-412, Korea.
Abstract
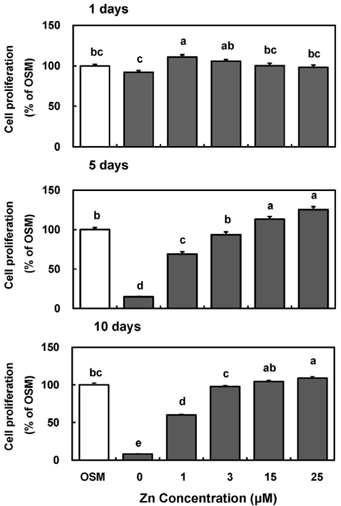
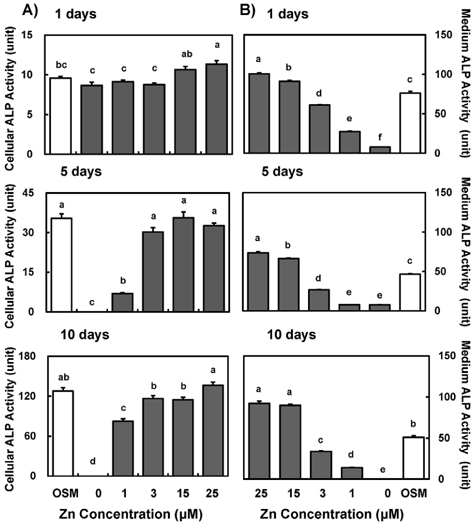
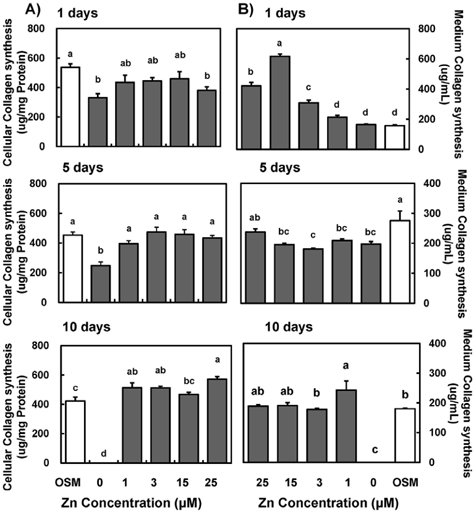
- Zinc is an essential trace element required for bone formation, however not much has been clarified yet for its role in osteoblast. We hypothesized that zinc would increase osteogenetic function in osteoblasts. To test this, we investigated whether zinc treatment enhances bone formation by stimulating osteoblast proliferation, bone marker protein alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. MC3T3-E1 cells were cultured and treated with various concentrations of zinc (0, 1, 3, 15, 25 uM) along with a normal osteogenic medium (OSM) as control for 1, 5, 10 days. As measured by MTT assay for mitochondrial metabolic activity, cell proliferation was stimulated even at low zinc treatment (1-3 micrometer) compared to OSM, and it was stimulated in a zinc concentration-dependent manner during 5 and 10 days, with the most pronounced effect at 15 and 25 uM Zn. Cellular (synthesized) alkaline phosphatase (ALP) activity was increased in a zinc concentration-dependent manner, so did medium (secreted) ALP activity. Cellular collagen concentration was increased by zinc as time went by, therefore with the maximum zinc stimulatory effect in 10 days, and medium collagen concentration showed the same pattern even on 1 and 5 day. This zinc stimulatory effect of collagen synthesis was observed in cell matrix collagen staining. The study results imply that zinc can increase osteogenic effect by stimulating cell proliferation, ALP activity and collagen synthesis in osteoblastic cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003. 423:349–355.
Article2. Noda M. Stein GS, Lian JB, editors. Cellular and molecular biology of bone. Molecular mechanisms mediating developmental and hormone-regulated expression of genes in osteoblasts: An integrated relationship of cell growth and differentiation. 1993. San Diego: Academic Press;48–97.3. Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999. 39:246–261.
Article4. Choi JY, Lee BH, Song KB, Park RW, Kim IS, Sohn KY, Jo JS, Ryoo HM. Expression patterns of bone-related proteins during osteoblastic differentiation in MC3T3-E1 cells. J Cell Biochem. 1996. 61:609–618.
Article5. Chien KR, Karsenty G. Longevity and lineages: toward the integrative biology of degenerative diseases in heart, muscle, and bone. Cell. 2005. 120:533–544.
Article6. Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003. 57:399–411.
Article7. Hsieh HS, Navia JM. Zinc deficiency and bone formation in guinea pig alveolar implants. J Nutr. 1980. 110:1581–1588.
Article8. Oner G, Bhaumick B, Bala RM. Effect of zinc deficiency on serum somatomedin levels and skeletal growth in young rats. Endocrinology. 1984. 114:1860–1863.
Article9. Yamaguchi M, Yamaguchi R. Action of zinc on bone metabolism in rats: increases in alkaline phosphatase activity and DNA content. Biochem Pharmacol. 1986. 35:773–777.10. Hall SL, Dimai HP, Farley JR. Effects of zinc on human skeletal alkaline phosphatase activity in vitro. Calcif Tissue Int. 1999. 64:163–172.
Article11. Yamaguchi M, Oishi H, Suketa Y. Stimulatory effect of zinc on bone formation in tissue culture. Biochem Pharmacol. 1987. 36:4007–4012.
Article12. Yamaguchi M. beta-Alanyl-L-histidinato zinc and bone resorption. Gen Pharmacol. 1995. 26:1179–1183.13. Yamaguchi M. Role of zinc in bone formation and bone resorption. Journal of Trace Elements in Experimental Medicine. 1998. 11:119–135.
Article14. Hashizume M, Yamaguchi M. Stimulatory effect of beta-alanyl-L-histidinato zinc on cell proliferation is dependent on protein synthesis in osteoblastic MC3T3-E1 cells. Mol Cell Biochem. 1993. 122:59–64.
Article15. Hashizume M, Yamaguchi M. Effect of beta-alanyl-L-histidinato zinc on differentiation of osteoblastic MC3T3-E1 cells: increases in alkaline phosphatase activity and protein concentration. Mol Cell Biochem. 1994. 131:19–24.
Article16. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992. 7:235–246.
Article17. Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990. 4:3111–3123.
Article18. Cao J, Bobo JA, Liuzzi JP, Cousins RJ. Effects of intracellular zinc depletion on metallothionein and ZIP2 transporter expression and apoptosis. J Leukoc Biol. 2001. 70:559–566.19. Bremner I, Beattie JH. Copper and zinc metabolism in health and disease: speciation and interactions. Proc Nutr Soc. 1995. 54:489–499.
Article20. Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001. 14:331–341.
Article21. Yamaguchi M, Matsui T. Stimulatory effect of zinc-chelating dipeptide on deoxyribonucleic acid synthesis in osteoblastic MC3T3-E1 cells. Peptides. 1996. 17:1207–1211.
Article22. Ma ZJ, Yamaguchi M. Role of endogenous zinc in the enhancement of bone protein synthesis associated with bone growth of newborn rats. J Bone Miner Metab. 2001. 19:38–44.
Article23. Ma ZJ, Misawa H, Yamaguchi M. Stimulatory effect of zinc on insulin-like growth factor-I and transforming growth factor-beta 1 production with bone growth of newborn rats. Int J Mol Med. 2001. 8:623–628.24. Magnusson P, Larsson L, Magnusson M, Davie MW, Sharp CA. Isoforms of bone alkaline phosphatase: characterization and origin in human trabecular and cortical bone. J Bone Miner Res. 1999. 14:1926–1933.
Article25. Peterson WJ. A method to assess the proliferative activity of small numbers of murine peripheral blood mononuclear cells. J Immunol Methods. 1987. 96:171–177.
Article26. Lian JB, Stein GS. Transcriptional control of vitamin D-regulated proteins. J Cell Biochem. 1992. 49:37–45.
Article27. Fernley HN, Walker PG. Inhibition of alkaline phosphatase by L-phenylalanine. Biochem J. 1970. 116:543–544.
Article28. Simmons DJ. Wallwork JC, Sandstead HH, editors. Nutrition and Bone Development. Zinc. 1990. New York: Oxford University Press;316–342.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- On the Activity of MC3T3-E1 Cell in vitro
- Effect of Centrifugal Force on Cellular Activity of Osteoblastic MC3T3-E1 Cells in vitro
- Glycyrrhiza uralensis (licorice) extracts increase cell proliferation and bone marker enzyme alkaline phosphatase activity in osteoblastic MC3T3-E1 cells
- Effects of Scytosiphon lomentaria on osteoblastic proliferation and differentiation of MC3T3-E1 cells
- Cellular zinc deficiency inhibits the mineralized nodule formation and downregulates bone-specific gene expression in osteoblastic MC3T3-E1 cells