Nutr Res Pract.
2010 Jun;4(3):203-207.
The effect of Oligonol intake on cortisol and related cytokines in healthy young men
- Affiliations
-
- 1Department of Physiology, College of Medicine, Soonchunhyang University, 366-1 Ssang yong-dong, Cheonan 331-946, Korea. syo99@nate.com
Abstract
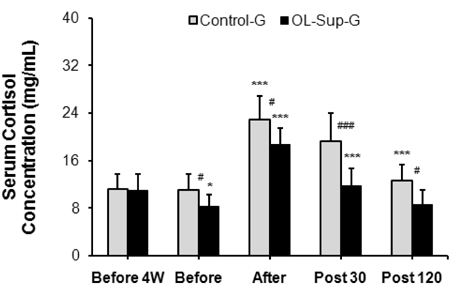
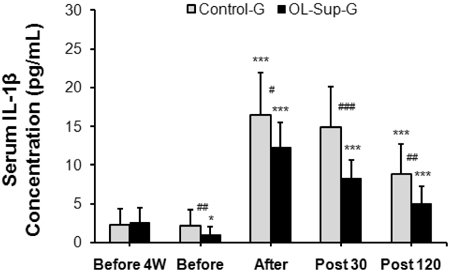
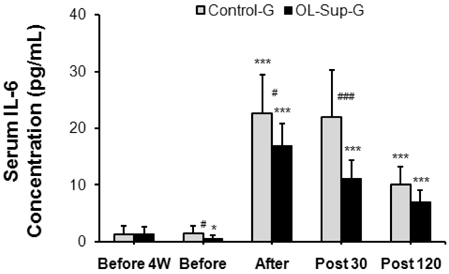
- This study investigated the effects of Oligonol intake on cortisol, interleukin (IL)-1beta, and IL-6 concentrations in the serum at rest and after physical exercise loading. Nineteen healthy sedentary male volunteers participated in this study. The physical characteristics of the subjects were: a mean height of 174.2 +/- 2.7 cm, a mean weight of 74.8 +/- 3.6 kg and a mean age of 22.8 +/- 1.3 years. Each subject received 0.5 L water with Oligonol (100 mg/day) (n = 10) or a placebo (n = 9) daily for four weeks. The body composition, the white blood cell (WBC) and differential counts as well as the serum cortisol, IL-1beta, and IL-6 concentrations were measured before and after Oligonol intake. The cortisol concentration and serum levels of IL-1beta and IL-6 after Oligonol intake were significantly decreased compared to before treatment (P < 0.01, respectively). In addition, the rate of increase of these factors after exercise was decreased compared to the placebo group. There was no change in the WBC and differential cell counts. These results suggest that oral Oligonol intake for four weeks had a significant effect on inhibition of inflammatory markers in healthy young men.
Keyword
MeSH Terms
Figure
Reference
-
1. Aruoma OI. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res. 2003. 523-524:9–20.
Article2. Owen RW, Giacosa A, Hull WE, Haubner R, Spiegelhalder B, Bartsch H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur J Cancer. 2000. 36:1235–1247.
Article3. Aruoma OI, Sun B, Fujii H, Neergheen VS, Bahorun T, Kang KS, Sung MK. Low molecular proanthocyanidin dietary biofactor oligonol: its modulation of oxidative stress, bioefficacy, neuroprotection, food application and chemoprevention potentials. Biofactors. 2006. 27:245–265.
Article4. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease and cancer. Pharmacol Rev. 2000. 52:673–751.5. Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005. 81:215S–217S.
Article6. Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolic as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res. 2005. 579:200–213.
Article7. Soobrattee MA, Bahorun T, Aruoma OI. Chemopreventive actions of polyphenolic compounds in cancer. Biofactors. 2006. 27:19–35.
Article8. Nakagawa T, Yokozawa T, Satoh A, Kim HY. Attenuation of renal ischemia-reperfusion injury by proanthocyanidin-rich extract from grape seeds. J Nutr Sci Vitaminol (Tokyo). 2005. 51:283–286.
Article9. Li MH, Jang JH, Sun B, Surh YJ. Protective effects of oligomers of grape seed polyphenols against b-amyloid-induced oxidative cell death. Ann N Y Acad Sci. 2004. 1030:317–329.
Article10. Fujii H, Yokozawa T, Kim YA, Tohda C, Nonaka G. Protective effect of grape seed polyphenols against high glucose-induced oxidative stress. Biosci Biotechnol Biochem. 2006. 70:2104–2111.
Article11. Fujii H, Sun B, Nishioka H, Hirose A, Aruoma OI. Evaluation of the safety and toxicity of the oligomerized polyphenol Oligonol. Food Chem Toxicol. 2007. 45:378–387.
Article12. Drenth JP, Van Uum SH, Van Deuren M, Pesman GJ, Van der Ven-Jongekrijg J, Van der Meer JW. Endurance run increases circulating IL-6 and IL-1ra but down regulates ex vivo TNF-α and IL-1β production. J Appl Physiol. 1995. 79:1497–1503.
Article13. Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. 1999. 515:287–291.
Article14. Belcastro AN, Arthur GD, Albisser TA, Raj DA. Heart, liver, and skeletal muscle myeloperoxidase activity during exercise. J Appl Physiol. 1996. 80:1331–1335.
Article15. Nishioka H, Fujii H, Sun B, Aruoma OI. Comparative efficacy of oligonol, catechin and (-)-epigallocatechin 3-O-gallate in modulating the potassium bromate-induced renal toxicity in rats. Toxicology. 2006. 226:181–187.
Article16. Tomobe K, Fujii H, Sun B, Nishioka H, Aruoma OI. Modulation of infection-induced inflammation and locomotive deficit and longevity in senescence-accelerated mice-prone (SAMP8) model by the oligomerized polyphenol oligonol. Biomed Pharmacother. 2007. 61:427–434.
Article17. Kundu JK, Chang EJ, Fujii H, Sun B, Surh YJ. Oligonol inhibits UVB-induced COX-2 expression in HR-1 hairless mouse skin: AP-1 and C/EBP as potential upstream targets. Photochem Photobiol. 2008. 84:399–406.
Article18. Sakurai T, Nishioka H, Fujii H, Nakano N, Kizaki T, Radak Z, Izawa T, Haga S, Ohno H. Antioxidative effects of a new lychee fruit-derived polyphenol mixture, oligonol, converted into a low-molecular form in adipocytes. Biosci Biotechnol Biochem. 2008. 72:463–476.
Article19. Northoff H, Berg A, Weinstock C. Similarities and differences of the immune response to exercise and trauma: the IFN-concept. Can J Physiol Pharmacol. 1998. 76:497–504.
Article20. Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007. 103:693–699.
Article21. Metz JR, Huising MO, Leon K, Verburg-van Kemenade BM, Flik G. Central and peripheral interleukin-β1 and interleukin-1 receptor 1 expression and their role in the acute stress response of common carp, Cyprinus carpio L. J Endocrinol. 2006. 191:25–35.
Article22. O'Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003. 991:123–132.23. Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991. 77:1627–1652.
Article24. Benjamin D, Sharma V, Kubin M, Klein JL, Sartori A, Holliday J, Trinchieri G. IL-12 expression in AIDS-related lymphoma B cell lines. J Immunol. 1996. 156:1626–1637.25. Kang K, Kubin M, Cooper KD, Lessin SR, Trinchieri G, Rook AH. IL-12 synthesis by human langerhans cells. J Immunol. 1996. 156:1402–1407.26. Kaplan A, Baeza M, Reddigari S, Kuna P. Histamine-releasing factors. Int Arch Allergy Appl Immunol. 1991. 94:148–153.
Article27. Cannon JG, Evans WJ, Hughes VA, Meredith CN, Dinarello CA. Physiological mechanisms contributing to increased interleukin-1 secretion. J Appl Physiol. 1986. 61:1869–1874.
Article28. Shephard RJ, Rhind S, Shek PN. Exercise and the immune system: natural killer cells, interleukins and related responses. Sports Med. 1994. 18:340–369.29. Tilg H, Dinarello CA, Mier JW. IL-6 and APPs: anti-inflammatory and immunosuppressive mediators. Immunol Today. 1997. 18:428–432.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oligonol promotes anti-aging pathways via modulation of SIRT1-AMPK-Autophagy Pathway
- Effects of Stress induced by Traffic Accidents on the Blood Concentrations of Cortisol, Glucose and Cytokines
- Protective role of oligonol from oxidative stress-induced inflammation in C6 glial cell
- Comparative Analysis of Salivary Cortisol in Young Adult Patients with Temporomandibular Disorders
- Preventive Effects of Oligomerized Polyphenol on Estradiol-Induced Prostatitis in Rats