Nutr Res Pract.
2010 Jun;4(3):183-190.
Protective effect of enzymatic hydrolysates from highbush blueberry (Vaccinium corymbosum L.) against hydrogen peroxide-induced oxidative damage in Chinese hamster lung fibroblast cell line
- Affiliations
-
- 1School of Food Technology and Nutrition, Chonnam National University, Yeosu 550-749, Korea.
- 2Department of Food Bioengineering, Jeju National University, Jeju 690-756, Korea.
- 3School of Marine Biomedical Science, Jeju National University, 1 Ara-dong, Jeju-si, Jeju 690-756, Korea. youjinj@jejunu.ac.kr
Abstract
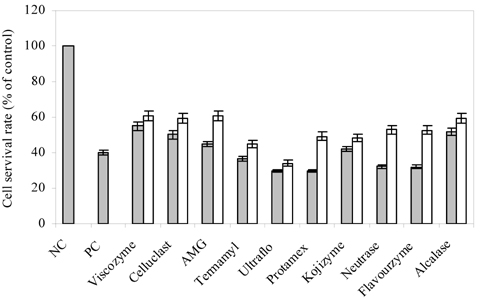
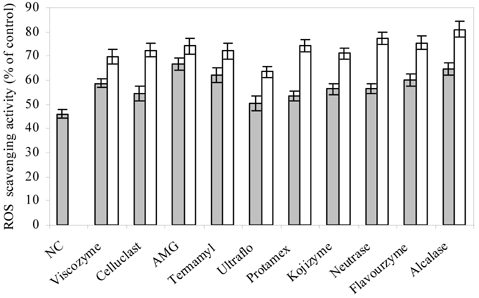
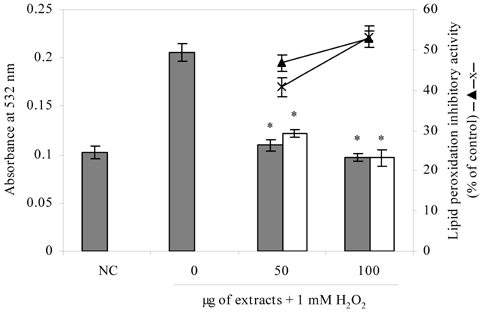
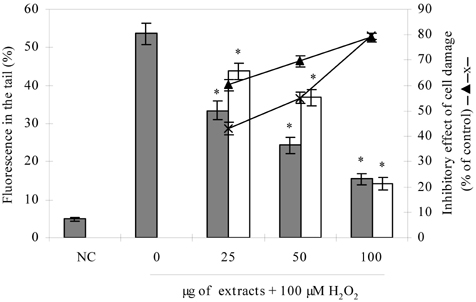
- Blueberry was enzymatically hydrolyzed using selected commercial food grade carbohydrases (AMG, Celluclast, Termamyl, Ultraflo and Viscozyme) and proteases (Alcalase, Flavourzyme, Kojizyme, Neutrase and Protamex) to obtain water soluble compounds, and their protective effect was investigated against H2O2-induced damage in Chinese hamster lung fibroblast cell line (V79-4) via various published methods. Both AMG and Alcalase hydrolysates showed higher total phenolic content as well as higher cell viability and ROS scavenging activities, and hence, selected for further antioxidant assays. Both AMG and Alcalase hydrolysates also showed higher protective effects against lipid peroxidation, DNA damage and apoptotic body formation in a dose-dependent fashion. Thus, the results indicated that water soluble compounds obtained by enzymatic hydrolysis of blueberry possess good antioxidant activity against H2O2-induced cell damage in vitro.
MeSH Terms
-
Animals
Asian Continental Ancestry Group
Blueberry Plant
Cell Line
Cell Survival
Cricetinae
Cricetulus
DNA Damage
Endopeptidases
Fibroblasts
Humans
Hydrogen
Hydrolysis
Lipid Peroxidation
Lung
Metalloendopeptidases
Peptide Hydrolases
Phenol
Subtilisins
Endopeptidases
Hydrogen
Metalloendopeptidases
Peptide Hydrolases
Phenol
Subtilisins
Figure
Reference
-
1. LaFerla FM. Calcium dyshomeostasis and intracellular signaling in Alzheimer's disease. Nat Rev Neurosci. 2002. 11:862–872.2. Prasad KN, Cole WC, Hovland AR, Prasad KC, Nahreini P, Kumar B, Edwards-Prasad J, Andreatta CP. Multiple antioxidants in the prevention and treatment of neurodegenerative disease: analysis of biologic rationale. Curr Opin Neurol. 1999. 12:761–770.
Article3. Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001. 64:43–51.
Article4. Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2001. 29:323–333.
Article5. Triggiani V, Resta F, Guastamacchia E, Sabba C, Licchelli B, Ghiyasaldin S, Tafaro E. Role of antioxidants, essential fatty acids, carnitine, vitamins, phytochemicals and trace elements in the treatment of diabetes mellitus and its chronic complications. Endocr Metab Immune Disord Drug Targets. 2006. 6:77–93.
Article6. De Flora S, Ferguson LR. Overview of mechanisms of cancer chemopreventive agents. Mutat Res. 2005. 591:8–15.
Article7. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007. 39:44–84.
Article8. Prior RL, Cao G, Martin A, Sofic E, McEwen J, O'Brien C, Lischner N, Ehlenfeldt M, Kalt W, Krewer G, Mainland CM. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J Agric Food Chem. 1998. 46:2686–2693.
Article9. Neto CC. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nut Food Res. 2007. 51:652–664.
Article10. Youdim KA, Shukitt-Hale B, Martin A, Wang H, Denisova N, Bickford PC, Joseph JA. Short-term dietary supplementation of blueberry polyphenolics: beneficial effects on aging brain performance and peripheral tissue function. Nutr Neurosci. 2000. 3:383–397.
Article11. Gruenwald J. PDR for Herbal Medicines. 2004. Newjersy: Thomson PDR publisher;228–235.12. Chambers B, Camire M. Can cranberry supplementation benefit adults with type 2 diabetes? Diabetes Care. 2003. 26:2695–2696.
Article13. Heo SJ, Lee KW, Song CB, Jeon YJ. Antioxidant activity of enzymatic extracts from brown seaweeds. Algae. 2003. 18:71–81.
Article14. Chandler SF, Dodds JH. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasidine in callus cultures of Solanum laciniatum. Plant Cell Rep. 1993. 2:105–110.15. Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989. 119:203–210.
Article16. Engelmann J, Volk J, Leyhausen G, Geurtsen W. ROS formation and glutathione levels in human oral fibroblasts exposed to TEGDMA and camphorquinone. J Biomed Mater Res Part B Appl Biomater. 2005. 75:272–276.
Article17. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.
Article18. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988. 175:184–191.
Article19. Gschwind M, Huber G. Apoptotic cell death induced by β-amyloid 1-42 peptide is cell type dependent. J Neurochem. 1995. 65:292–300.
Article20. Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Curr Opin Lipidol. 2005. 16:77–84.
Article21. Schmidt BM, Howell AB, Mceniry B, Knight CT, Seigler D, Erdman JW Jr, Lila MA. Effective separation of potent antiproliferation and antiadhesion components from wild blueberry (Vaccinium angustifolium Ait.) fruits. J Agric Food Chem. 2004. 52:6433–6442.
Article22. Yi W, Akoh CC, Fischer J, Krewer G. Effects of phenolic compounds in blueberries and muscadine grapes on HepG2 cell viability and apoptosis. Food Res Int. 2006. 39:628–638.
Article23. Sellappan S, Akoh CC, Krewer G. Phenolic compounds and antioxidant capacity of Georgia-Grown blueberries and blackberries. J Agric Food Chem. 2002. 50:2432–2438.
Article24. Rhee SG. Redox signaling: hydrogen peroxide as intracellular messenger. Exp Mol Med. 1999. 31:53–59.
Article25. Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 1989. Oxford: Oxford University Press;96–98.26. Wang H, Joseph JA. Mechanisms of hydrogen peroxide-induced calcium dysregulation in PC12 cells. Free Radic Biol Med. 2000. 28:1222–1231.
Article27. Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998. 141:1423–1432.
Article28. Castillo MR, Babson JR. Ca2+-dependent mechanisms of cell injury in cultured cortical neurons. Neuroscience. 1998. 86:1133–1144.
Article29. McGinnis KM, Wang KKW, Gnegy ME. Alterations of extracellular calcium elicit selective modes of cell death and protease activation in SH-SY5Y human neuroblastoma cells. J Neurochem. 1999. 72:1853–1863.
Article30. Mansouri A, Makris DP, Kefalas P. Determination of hydrogen peroxide scavenging activity of cinnamic and benzoic acids employing a highly sensitive peroxyoxalate chemiluminescence-based assay: Structure-activity relationships. J Pharm Biomed Anal. 2005. 39:22–26.
Article31. Yoshikawa T, Naito Y, Kondo M. Hiramatsu M, Yoshikawa T, Inoue M, editors. Food and disease. Free radicals and diseases. 1997. New York: Plenum Press;11–19.32. Halliwell B, Gutteridge MC. Oxygen toxicity, oxygen radical, transition metals and disease. Biochem J. 1984. 219:1–14.33. Chaudhary AK, Nokubo M, Reddy GR, Yeola SN, Morrow JD, Blair IA, Marnett LJ. Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science. 1994. 265:1580–1582.
Article34. Kassie F, Parzefall W, Knasmuller S. Single cell gel electrophoresis assay: a new technique for human biomonitoring studies. Mutat Res. 2000. 463:13–31.
Article35. Wilms LC, Hollman PCH, Boots AW, Kleinjans JCS. Protection by quercetin and quercetin-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutat Res. 2005. 582:155–162.
Article36. Cozzi R, Ricordy R, Aglitti T, Gatta V, Perticone P, De Salvia R. Ascorbic acid and beta-carotene as modulators of oxidative damage. Carcinogenesis. 1997. 18:223–228.
Article37. Jang JH, Surh YJ. Protective effects of resveratrol on hydrogen peroxide-induced apoptosis in rat pheochromocytoma (PC12) cells. Mutat Res. 2001. 496:181–190.
Article38. Kerr JF, Gobe GC, Winterford CM, Harmon BV. Schwartz LM, Osborne BA, editors. Anatomical methods in cell death. Methods in cell biology. 1995. New York: Academic Press;1–27.39. Vicki S, Peter AH, Philip JC. Brady JM, editor. Detecting and quantifying apoptosis in tissue sections. Apoptosis methods and protocols. 2004. New Jersey: Humana Press;67–84.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of propofol and etomidate on hydrogen peroxide-induced oxidative damage in hepatocyte
- Effect of Hydrogen Peroxide-induced Oxidative Stress on the Senescence of Trabecular Meshwork Cells
- Occurrence of Anthracnose on Highbush Blueberry Caused by Colletotrichum Species in Korea
- Protective Effect of Yellow-Green Vegetable Juices on DNA Damage in Chinese Hamster Lung Cell Using Comet Assay
- The Effect of Induced Heat Shock Protein 33 in Human Corneal Epithelial Cell