Nutr Res Pract.
2010 Apr;4(2):93-98.
Protection by Chrysanthemum zawadskii extract from liver damage of mice caused by carbon tetrachloride is maybe mediated by modulation of QR activity
- Affiliations
-
- 1Department of Animal Science and Biotechnology and School of Applied Biosciences, Kyungpook National University, 1370 Sankyuk-dong, Buk-gu, Daegu 702-701, Korea. vision@knu.ac.kr
- 2Department of Food Science and Nutrition, Hallym University, Chuncheon 200-702, Korea.
- 3Department of Human Ecology, KyungSung University, Busan 608-736, Korea.
Abstract
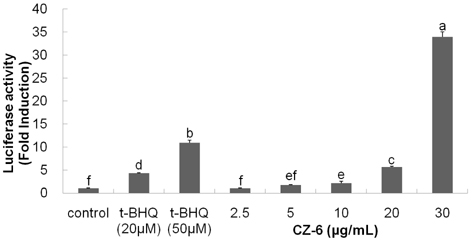
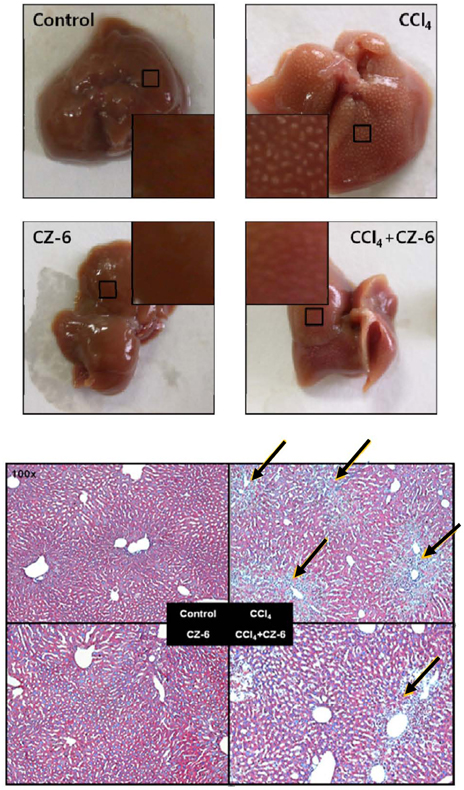
- Our previous study demonstrated that methanolic extract of Chrysanthemum zawadskii Herbich var. latilobum Kitamura (Compositae) has the potential to induce detoxifying enzymes such as NAD(P)H:(quinone acceptor) oxidoreductase 1 (EC 1.6.99.2) (NQO1, QR) and glutathione S-transferase (GST). In this study we further fractionated methanolic extract of Chrysanthemum zawadskii and investigated the detoxifying enzyme-inducing potential of each fraction. The fraction (CZ-6) shown the highest QR-inducing activity was found to contain (+)-(3S,4S,5R,8S)-(E)-8-acetoxy-4-hydroxy-3-isovaleroyloxy-2-(hexa-2,4-diynyliden)-1,6-dioxaspiro [4,5] decane and increased QR enzyme activity in a dose-dependent manner. Furthermore, CZ-6 fraction caused a dose-dependent enhancement of luciferase activity in HepG2-C8 cells generated by stably transfecting antioxidant response element-luciferase gene construct, suggesting that it induces antioxidant/detoxifying enzymes through antioxidant response element (ARE)-mediated transcriptional activation of the relevant genes. Although CZ-6 fraction failed to induce hepatic QR in mice over the control, it restored QR activity suppressed by CCl4 treatment to the control level. Hepatic injury induced by CCl4 was also slightly protected by pretreatment with CZ-6. In conclusion, although CZ-6 fractionated from methanolic extract of Chrysanthemum zawadskii did not cause a significant QR induction in mice organs such as liver, kidney, and stomach, it showed protective effect from liver damage caused by CCl4.
Keyword
MeSH Terms
-
Alkanes
Animals
Antioxidant Response Elements
Carbon
Carbon Tetrachloride
Chrysanthemum
Glutathione Transferase
Kidney
Liver
Luciferases
Methanol
Mice
NAD(P)H Dehydrogenase (Quinone)
Stomach
Transcriptional Activation
Alkanes
Carbon
Carbon Tetrachloride
Glutathione Transferase
Luciferases
Methanol
NAD(P)H Dehydrogenase (Quinone)
Figure
Reference
-
1. Kwon HS, Ha TJ, Hwang SW, Jin YM, Nam SH, Park KH, Yang MS. Cytotoxic flavonoids from the whole plants of Chrysanthemum zawadskii Herbich var. latilobum Kitamura. J Life Sci. 2006. 16:746–749.
Article2. Kang HS, Park MJ, Jin KS, Kim YH, Jun M, Lim HJ, Jo WK, Kim JS, Jeong WS. Regulatory roles of Chrysanthemum zawadskii roots in nuclear factor E2-related factor 2/antioxidant response element pathway. Food Sci Biotechnol. 2008. 17:367–372.3. Kim JR, Kim JH, Lim HA, Jang CH, Kim JH, Kwon CS, Kim YK, Kim JS. Induction of quinone reductase, an anticarcinogenic marker enzyme, by extract from Chrysanthemum zawadskii var. latilobum K. Journal of Food Science and Nutrition. 2005. 10:340–343.
Article4. Mehta RG, Pezzuto JM. Discovery of cancer preventive agents from natural products: from plants to prevention. Curr Oncol Rep. 2002. 4:478–486.
Article5. Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003. 371:887–895.
Article6. Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, Yamamoto M, Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002. 99:11908–11913.
Article7. Prochaska HJ, De Long MJ, Talalay P. On the mechanisms of induction of cancer-protective enzymes: a unifying proposal. Proc Natl Acad Sci U S A. 1985. 82:8232–8236.
Article8. Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992. 89:2399–2403.
Article9. Kim BR, Hu R, Keum YS, Hebbar V, Shen G, Nair SS, Kong AN. Effects of glutathione on antioxidant response element-mediated gene expression and apoptosis elicited by sulforaphane. Cancer Res. 2003. 63:7520–7525.10. Benson AM, Hunkeler MJ, Talalay P. Increase of NAD(P) H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980. 77:5216–5220.
Article11. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951. 193:265–275.
Article12. Proctor E, Chatamra K. High yield micronodular cirrhosis in the rat. Gastroenterology. 1982. 83:1183–1190.
Article13. Yin H-Q, Lee BW, Kim YC, Sohn DH, Lee BH. Induction of the anticarcinogenic marker enzyme, quinone reductase, by Dalbergiae Lignum. Arch Pharm Res. 2004. 27:919–922.
Article14. Kim JH, Lee JS, Song KS, Kwon CS, Kim YK, Kim JS. Polyozellin isolated from Polyozellus multiplex induces phase 2 enzymes in mouse hepatoma cells and differentiation in human myeloid leukaemic cell lines. J Agric Food Chem. 2004. 52:451–455.
Article15. Seo JY, Park J, Kim HJ, Lee IA, Lim JS, Lim SS, Choi SJ, Park JH, Kang HJ, Kim JS. Isoalantolactone from Inula helenium caused Nrf2-mediated induction of detoxifying enzymes. J Med Food. 2009. 12:1038–1045.
Article16. Seo JY, Lim SS, Kim JR, Lim JS, Ha YR, Lee IA, Kim EJ, Park JH, Kim JS. Nrf2-mediated induction of detoxifying enzymes by alantolactone present in Inula helenium. Phytother Res. 2008. 22:1500–1505.
Article17. Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997. 236:313–322.
Article18. Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, Eguchi M, Wada Y, Kumagai Y, Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol. 2009. 29:493–502.
Article19. Wong F, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998. 153:109–118.
Article20. Albano E, Tomasi A, Goria-Gatti L, Poli G, Vannini V, Dianzani MU. Free radical metabolism of alcohols by rat liver microsomes. Free Radic Res Commun. 1987. 3:243–249.
Article21. Connor HD, Lacagnin LB, Knecht KT, Thurman RG, Mason RP. Reaction of glutathione with a free radical metabolite of carbon tetrachloride. Mol Pharmacol. 1990. 37:443–451.22. McCay PB, Lai EK, Poyer JL, DuBose CM, Janzen EG. Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism: Observation of lipid radicals in vivo and in vitro. J Biol Chem. 1984. 259:2135–2143.
Article23. Packer JE, Slater TF, Willson RL. Reactions of the carbon tetrachloride-related peroxy free radical (CCl3O2.) with amino acids: Pulse radiolysis evidence. Life Sci. 1978. 23:2617–2620.
Article24. Slater TF. Free-radical mechanisms in tissue injury. Biochem J. 1984. 222:1–15.
Article25. Recknagel RO. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967. 19:145–208.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-oxidant activities of kiwi fruit extract on carbon tetrachloride-induced liver injury in mice
- Effect of Chrysanthemum zawadskii Extract on Dermal Papilla Cell Proliferation and Hair Growth
- Synergetic Hepatoprotective Effects of Korean Red Ginseng and Pueraria Radix on the Liver Damaged-Induced by Carbon Tetrachloride (CClâ‚„) in Mice
- Influence of Hepatic Damage on the Male Reproductive Glands: II. Influence of Hepatic Damage on the Effect of Exogenous Sex Hormones on the Reproductive Glands of White Rats
- Influence of Hepatic Damage on the Male Reproductive Glands: IV. Effect of Cortisone on the Reproductive Glands of Male White Rats with Liver Damage