Nutr Res Pract.
2009 Sep;3(3):185-191.
Modulatory effects of alpha- and gamma-tocopherols on 4-hydroxyestradiol induced oxidative stresses in MCF-10A breast epithelial cells
- Affiliations
-
- 1Department of Food and Nutrition, Sookmyung Women's University, 52 Hyochangwon-gil, Yongsan-gu, Seoul 140-742, Korea. mksung@sm.ac.kr
- 2National Cancer Center Institute, 111 Jungbalsan-ro, Ilsandong-gu, Goyang, Gyeonggi 410-769, Korea.
- 3Department of Surgery, College of Medicine and Asan Medical Center, 388-1 Pungnap-2 dong, Songpa-gu, Seoul 138-736, Korea.
Abstract
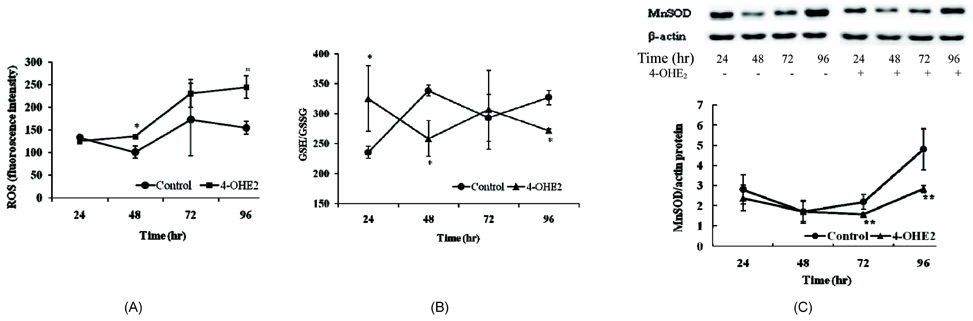
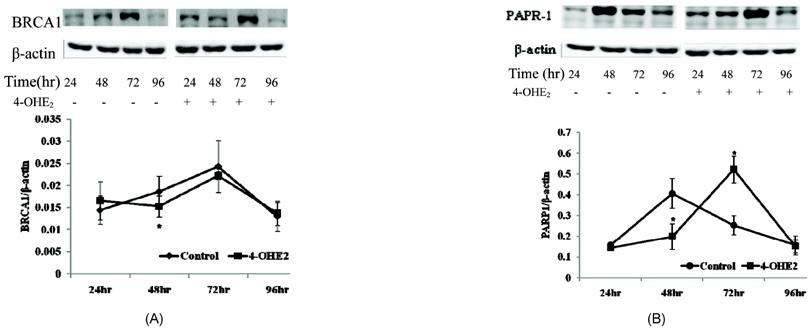
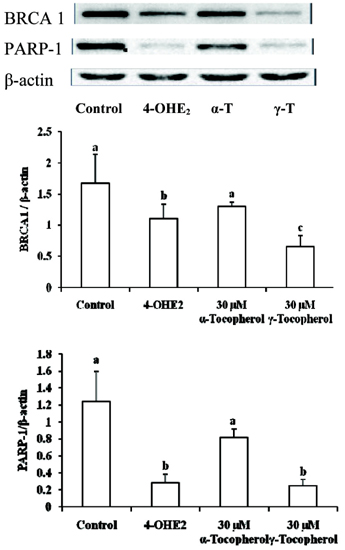
- The elevated level of circulating estradiol increases the risk of breast tumor development. To gain further insight into mechanisms involved in their actions, we investigated the molecular mechanisms of 4-hydroxyestradiol (4-OHE2) to initiate and/or promote abnormal cell growth, and of alpha- or gamma-tocopherol to inhibit this process. MCF-10A, human breast epithelial cells were incubated with 0.1 microM 4-OHE2, either with or without 30 microM tocopherols for 96 h. 4-OHE2 caused the accumulation of intracellular ROS, while cellular GSH/GSSG ratio and MnSOD protein levels were decreased, indicating that there was an oxidative burden. 4-OHE2 treatment also changed the levels of DNA repair proteins, BRCA1 and PARP-1. gamma-Tocopherol suppressed the 4-OHE2-induced increases in ROS, GSH/GSSG ratio, and MnSOD protein expression, while alpha-tocopherol up-regulated BRCA1 and PARP-1 protein expression. In conclusion, 4-OHE2 increases oxidative stress reducing the level of proteins related to DNA repair. Tocopherols suppressed oxidative stress by scavenging ROS or up-regulating DNA repair elements.
MeSH Terms
Figure
Reference
-
1. Abiaka C, Al-Awadi F, Gulshan S, Al-Sayer H, Behbehani A, Farghaly M, Simbeye A. Plasma concentrations of alpha-tocopherol and urate in patients with different types of cancer. J Clin Pharm Ther. 2001. 26:265–270.
Article2. Aiub CA, Pinto LF, Felzenszwalb I. DNA-repair genes and vitamin E in the prevention of N-nitrosodiethylamine mutagenicity. Cell Biol Toxicol. 2009. 25:393–402.
Article3. Ame JC, Spenlehauer C, de Murcia G. The PARP superfamily. Bioessays. 2004. 26:882–893.
Article4. Arrigo AP. Gene expression and the thiol redox state. Free Radic Biol Med. 1999. 27:936–944.
Article5. Baba T, Shimizu T, Suzuki Y, Ogawara M, Isono K, Koseki H, Kurosawa H, Shirasawa T. Estrogen, insulin, and dietary signals cooperatively regulate longevity signals to enhanceresistance to oxidative stress in mice. J Biol Chem. 2005. 280:16417–16426.
Article6. Bae I, Fan S, Meng Q, Rih JK, Kim HJ, Kang HJ, Xu J, Goldberg ID, Jaiswal AK, Rosen EM. BRCA1 induces antioxidant gene expression and resistance to oxidative stress. Cancer Res. 2004. 64:7893–7909.
Article7. Belous AR, Hachey DL, Dawling S, Roodi N, Parl FF. Cytochrome P450 1B1-mediated estrogen metabolism results in estrogen-deoxyribonucleoside adduct formation. Cancer Res. 2007. 67:812–817.
Article8. Brodie SG, Deng CX. BRCA1-associated tumorigenesis: what have we learned from knockout mice? Trends Genet. 2001. 17:S18–S22.
Article9. Campbell SE, Stone WL, Lee S, Whaley S, Yang H, Qui M, Goforth P, Sherman D, McHaffie D, Krishnan K. Comparative effects of RRR-alpha- and RRR-gamma-tocopherol on proliferation and apoptosis in human colon cancer cell lines. BMC Cancer. 2006. 6:13.
Article10. Campbell SE, Stone WL, Whaley SG, Qui M, Krishnan K. Gamma (gamma) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (gamma) expression in SW 480 human colon cancer cell lines. BMC Cancer. 2003. 3:25.11. Cantor SB, Andreassen PR. Assessing the link between BACH1 and BRCA1 in the FA pathway. Cell Cycle. 2006. 5:164–167.
Article12. Chen ZH, Na HK, Hurh YJ, Surh YJ. 4-Hydroxyestradiol induces oxidative stress and apoptosis in human mammary epithelial cells: possible protection by NF-kappaB and ERK/MAPK. Toxicol Appl Pharmacol. 2005. 208:46–56.
Article13. Colditz GA. Relationship between estrogen levels, use of hormone replacement therapy, and breast cancer. J Natl Cancer Inst. 1998. 90:814–823.
Article14. Cuendet M, Bolton JL. Response of human mammary epithelial cells to DNA damage induced by 4-hydroxyequilenin: Lack of p53-mediated G1 arrest. Chem Biol Interact. 2006. 161:271–278.
Article15. de Murcia G, Ménissier de Murcia J. Poly (ADP-ribose)polymerase: a molecular nick-sensor. Trends Biochem Sci. 1994. 19:172–176.16. De Soto JA, Deng CX. PARP-a inhibitor: are they the lomg-sought genetically specific drugs for BRCA1/2-associated breast cancer? Int J Med Sci. 2006. 3:117–123.17. Deng CX, Wang RH. Roles of BRCA1 in DNA damage repair: a link between development and cancer. Hum Mol Genet. 2003. 12 Spec No 1:R113–R123.
Article18. Esworthy RS, Baker MA, Chu FF. Expression of selenium-dependent glutathione peroxidase in human breast tumor cell lines. Cancer Res. 1995. 55:957–962.19. Fernandez SV, Russo IH, Russo J. Estradiol and its metabolites 4-hydroxyestradiol and 2-hydroxyestradiol inducemutation in human breast epithelial cells. Int J Cancer. 2006. 118:1862–1868.
Article20. Ginestier C, Liu S, Wicha MS. Getting to the root of BRCA1-deficient breast cancer. Cell Stem Cell. 2009. 5:229–330.
Article21. Goodman GE, Schaffer S, Omenn GS, Chen C, King I. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from beta-carotene andretinol efficacy trial. Cancer Epidemiol Biomarkers Prev. 2003. 12:518–526.22. Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980. 106:207–212.
Article23. Grodstein F, Stampfer MJ, Colditz GA, Willett WC, Manson JE, Joffe M, Rosner B, Fuchs C, Hankinson SE, Hunter DJ, Hennekens CH, Speizer FE. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997. 336:1769–1775.
Article24. Gu X, Song X, Dong Y, Cai H, Walters E, Zhang R, Pang X, Xie T, Guo Y, Sridhar R, Califano JA. Vitamin E succinate induces ceramide-mediated apoptosis in head and neck squamouscell carcinoma in vitro and in vivo. Clin Cancer Res. 2008. 14:1840–1848.
Article25. Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002. 16:1952–1954.
Article26. Han X, Liehr JG. 8-Hydroxylation of guanine bases in kidney and liver DNA of hamsters treated with estradiol: role of free radicals in estrogen-induced carcinogenesis. Cancer Res. 1994. 54:5515–5517.27. Harvell DM, Strecker TE, Tochacek M, Xie B, Pennington KL, McComb RD, Roy SK, Shull JD. Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proc Natl Acad Sci U S A. 2000. 97:2779–2784.
Article28. Hitchler MJ, Wikainapakul K, Yu L, Powers K, Attatippaholkun W, Domann FE. Epigenetic regulation of manganese superoxide dismutase expression in human breast cancer cells. Epigenetics. 2006. 1:163–171.
Article29. Jiang LJQ, Shienaga MK, Shigeno T, Christen S, Ames BN. Gamma-tocopherol supplementation inhibits protien nitration and ascorbate oxidation in rats with inflammation. Free Rad Biol Med. 2002. 33:1534–1542.
Article30. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003. 302:643–646.
Article31. Lane DP. Cancer. p53, guardian of the genome. Nature. 1992. 358:15–16.32. Li D, Saldeen T, Romeo F, Mehta JL. Relative Effects of alpha- and gamma-Tocopherol on Low-Density Lipoprotein Oxidation and Superoxide Dismutase and Nitric Oxide Synthase Activity and Protein Expression in Rats. J Cardiovasc Pharmacol Ther. 1999. 4:219–226.
Article33. Li JJ, Oberley LW, Fan M, Colburn NH. Inhibition of AP-1 and NF-kappaB by manganese-containing superoxide dismutase in human breast cancer cells. FASEB J. 1998. 12:1713–1723.
Article34. Liu R, Oberley TD, Oberley LW. Transfection and expression of MnSOD cDNA decreases tumor malignancy of human oral squamous carcinoma SCC-25 cells. Hum Gene Ther. 1997. 8:585–595.
Article35. Liu R, Oberley TD, Oberley LW. Effects of endothelial nitric oxide synthase gene expression on the tumor biology of human oral carcinoma SCC-25 cells. Cell Growth Differ. 1998. 9:239–246.36. Malkin D, Li FP, Strong LC, Fraumeni JF, Nelson CE, Kim DH, Kassel J, Gryka MA, Bischoff FZ, Tainsky MA. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990. 250:1233–1238.
Article37. McMillan DC, Talwar D, Sattar N, Underwood M, O'Reilly DS, McArdle C. The relationship between reduced vitamin antioxidant concentrations and the systemic inflammatory response in patients with common solid tumours. Clin Nutr. 2002. 21:161–164.
Article38. Mense SM, Singh B, Remotti F, Liu X, Bhat HK. Vitamin C and alpha-naphthoflavone prevent estrogen-induced mammary tumors and decrease oxidative stress in female ACI rats. Carcinogenesis. 2009. 30:1202–1208.
Article39. Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: Implication in redox and detoxification. Clin Chim Acta. 2003. 333:19–39.
Article40. Plummer ER. Inhibition of poly (ADP-ribose) polymerase in cancer. Curr Opin Pharmacol. 2006. 6:364–368.41. Rosen EM, Fan S, Pestell RG, Goldberg ID. BRCA1 gene in breast cancer. J Cell Physiol. 2003. 196:19–41.
Article42. Roy S, Lado BH, Khanna S, Sen CK. Vitamin E sensitive genes in the developing rat fetal brain: a high-density oligonucleotide microarray analysis. FEBS Lett. 2002. 530:17–23.
Article43. Service RF. New role for estrogen in cancer? Science. 1998. 279:1631–1633.
Article44. Theriault A, Chao JT, Wang Q, Gapor A, Adeli K. Tocotrienol: a review of its therapeutic potential. Clin Biochem. 1999. 32:309–319.
Article45. Tong WM, Yang YG, Cao WH, Galendo D, Frappart L, Shen Y, Wang ZQ. Poly (ADP-ribose) polymerase-1 plays a role in suppressing mammary tumorigenesis in mice. Oncogene. 2007. 26:3857–3867.
Article46. Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002. 108:171–182.
Article47. Walsh T, Casadei S, Coats KH, Swisher E, Stray SM, Higgins J, Roach KC, Mandell J, Lee MK, Ciernikova S, Foretiva L, Soucek PKM. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in Families at high risk of breast cancer. JAMA. 2007. 295:1379–1388.
Article48. Wittmann BM, Fujinaga K, Deng H, Ogba N, Montano MM. The breast cell growth inhibitor, estrogen down regulated gene 1, modulates a novel functional interaction between estrogen receptor alpha and transcriptional elongation factor cyclin T1. Oncogene. 2005. 24:5576–5588.
Article49. Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996. 36:203–232.
Article50. Yu W, Sanders BG, Kline K. RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells involves Bax translocation to mitochondria. Cancer Res. 2003. 63:2483–2491.51. Zhang J, Powell SN. The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res. 2005. 3:531–539.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptotic Effects of 6-Gingerol in Human Breast Cancer Cells
- Alfa - difluoromethylornithine Reduced Protein Phosphorylation in MCF-7 Human Breast Cancer Cells
- Breast Cancer Selective Disruption of Actin Cytoskeleton by Diallyl Trisulfide
- Effects of Polyamines on TNFalpha- or Tamoxifen-induced Apoptosis in Human Breast Cancer Cells
- Silibinin Enhances Ultraviolet B-Induced Apoptosis in MCF-7 Human Breast Cancer Cells