Nutr Res Pract.
2009 Jun;3(2):77-83.
Effects of retinoic acid isomers on apoptosis and enzymatic antioxidant system in human breast cancer cells
- Affiliations
-
- 1Department of Food and Nutrition, Yonsei University, 134 Shinchon-dong, Seodaemun-gu, Seoul 120-749, Korea. ycleekim@yonsei.ac.kr
Abstract
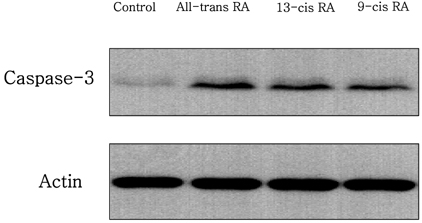
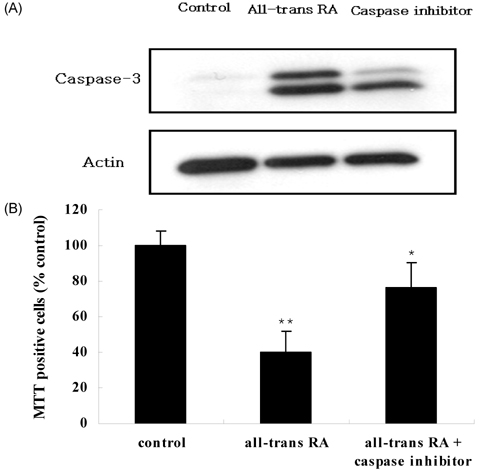
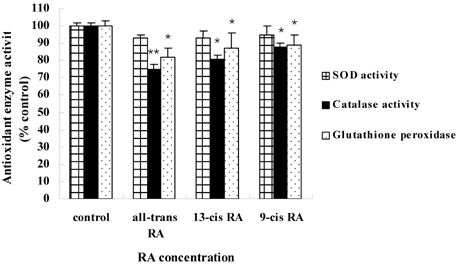
- Retinoic acids (RAs) modulate growth, differentiation, and apoptosis in normal, pre-malignant & malignant cells. In the present study, the effects of RA isomers (all-trans RA, 13-cis RA, and 9-cis RA) on the cell signal transduction of human breast cancer cells have been studied. The relationship between RAs and an enzymatic antioxidant system was also determined. Estrogen-receptor (ER) positive MCF-7 and ER-negative MDA-MB-231 human breast cancer cells were treated with different doses of each RA isomers, all-trans RA, 13-cis RA, or 9-cis RA. Treatment of RA isomers inhibited cell viability and induced apoptosis of MCF-7 cells as a result of increased caspase activity in cytoplasm and cytochrome C released from mitochondria. All-trans RA was the most effective RA isomer in both cell growth inhibition and induction of apoptosis in MCF-7 cells. However, no significant effect of RA isomers was observed on the cell growth or apoptosis in ER-negative MDA-MB-231 cells. In addition, activities of antioxidant enzymes such as catalase and glutathione peroxidase were decreased effectively after treatment of RA in MCF-7 cells, whereas SOD activity was rarely affected. Thus, the present data suggest that all-trans RA is the most potential inducer of apoptosis and modulator of antioxidant enzymes among RA isomers in MCF-7 human breast cancer cells.
MeSH Terms
Figure
Reference
-
1. Ahlemeyer B, Bauerbach E, Plath M, Steuber M, Heers C, Tegtmeier F, Krieglstein J. Retinoic acid reduces apoptosis and oxidative stress by preservation of SOD protein level. Free Radic Biol Med. 2001. 30:1067–1077.
Article2. Arruda VR, Salles TS, Costa FF, Saad ST. Glutathione peroxidase, reduced glutathione, superoxide dismutase and catalse in red cells of patients with hairy cell leukemia. Neoplasia. 1996. 43:99–102.3. Bai J, Rodriguez AM, Melendez JA, Cederbaum AI. Overexpression of catalase in cytosolic or mitochondrial compartment protects HepG2 cells against oxidative injury. J Biol Chem. 1999. 274:26217–26224.
Article4. Brodowicz T, Wiltschke C, Kandioler-Eckersberger D, Grunt TW, Rudas M, Schneider SM, Hejna M, Budinsky A, Zielinski CC. Inhibition of proliferation and induction of apoptosis in soft tissue sarcoma cells by interferon-alpha and retinoids. Br J Cancer. 1999. 80:1350–1358.
Article5. Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996. 10:940–954.
Article6. Conte da Frota ML Jr, Comes da Silva E, Behr GA, Roberto de Oliveira M, Dal Pizzol F, Klamt F, Moreira JC. All trans retinoic acid induces free radical generation and modulate antioxidant enzyme activities in rat sertoli cells. Mol Cell Biochem. 2006. 285:173–179.
Article7. Dietze EC, Caldwell LE, Marcom K, Collins SJ, Yee L, Swisshelm K, Hobbs KB, Bean GR, Seewaldt VL. Retinoids and retinoic acid receptors regulate growth arrest and apoptosis in human mammary epithelial cells and modulate expression of CBP/p300. Microsc Res Tech. 2002. 59:23–40.
Article8. Dimberg A, Oberg F. Retinoic acid-induced cell cycle arrest of human myeloid cell lines. Leuk Lymphoma. 2003. 44:1641–1650.
Article9. Drisko JA, Chapman J, Hunter VJ. The use of antioxidant therapies during chemotherapy. Gynecol Oncol. 2003. 88:434–439.
Article10. Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003. 22:7305–7315.
Article11. Gallagher RE. Retinoic acid resistance in acute promyelocytic leukemia. Leukemia. 2002. 16:1940–1958.
Article12. Gronemeyer H, Miturski R. Molecular mechanisms of retinoid action. Cell Mol Biol Lett. 2001. 6:3–52.13. Hara M, Suzuki S, Mori J, Yamashita K, Kumagai M, Sakuma T, Kakizawa T, Takeda T, Miyamoto T, Ichikawa K, Hashizume K. Thyroid hormone regulation of apoptosis induced by retinoic acid in promyeloleukemic HL-60 cells: studies with retinoic acid receptor-specific and retinoid x receptor-specific ligands. Thyroid. 2000. 10:1023–1034.
Article14. Hayashi K, Yokozaki H, Naka K, Yasui W, Lotan R, Tahara E. Overexpression of retinoic acid receptor beta induces growth arrest and apoptosis in oral cancer cell lines. Jpn J Cancer Res. 2001. 92:42–50.
Article15. Hengartner MO. The biochemistry of apoptosis (review). Nature. 2000. 407:770–776.16. Kuida K. Caspase-9. Int J Biochem Cell Biol. 2000. 32:121–124.
Article17. Kuypers FA, Lewis RA, Hua M, Schott MA, Discher D, Ernst JD, Lubin BH. Detection of altered membrane phospholipids asymmetry in subpopulations of juman red blood cells using fluorescently labeled annexin V. Blood. 1996. 87:1179–1187.
Article18. Manor D, Shmidt EN, Budhu A, Flesken-Nikitin A, Zgola M, Page R, Nikitin AY, Noy N. Mammary carcinoma suppressionby cellular retinoic acid binding protein-II. Cancer Res. 2003. 63:4426–4433.19. Marill J, Idres N, Capron CC, Nguyen E, Chabot GG. Retinoic acid metabolism and mechanism of action: a review. Curr Drug Metab. 2003. 4:1–10.
Article20. Melino G, Thiele CJ, Knight RA, Piacentini M. Retinoids and the control of growth/death decisions in human neuroblastoma cell lines. J Neurooncol. 1997. 31:65–83.21. Nagase M, Alam MM, Tsushima A, Yoshizawa T, Sakato N. Apoptosis induction by T-2 toxin: activation of caspase-9, caspase-3 and DFF-40/CAD through cytosolic release of cytochrome C in HL-60 cells. Biosci Biotechnol Biochem. 2001. 65:1741–1747.
Article22. Nagpal S, Chandraratna RA. Recent developments in receptor-selective retinoids. Curr Pharm Des. 2000. 6:919–931.
Article23. Napoli JL. Retinoic acid biosynthesis and metabolism. FASEB J. 1996. 10:993–1001.
Article24. Nelson AM, Gilliland KL, Cong Z, Thiboutot DM. 13-cis retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J Invest Dermatol. 2006. 126:2178–2189.
Article25. Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001. 31:1287–1312.
Article26. Ott DB, Lachance PA. Retinoic acid-a review. Am J Clin Nutr. 1979. 32:2522–2531.
Article27. Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007. 12:913–922.
Article28. Paik J, Blaner WS, Sommer KM, Moe R, Swisshlem K. Retinoids, retinoic acid receptors, and breast cancer. Cancer Invest. 2003. 21:304–312.
Article29. Pepper C, Ali K, Thomas A, Hoy T, Fegan C, Chowdary P, Kell J, Bentley P. Retinoid-induced apoptosis in B-cell chronic lymphocytic leukaemia cells is mediated through caspase-3 activation and is independent of p53, the retinoic acid receptor, and differentiation. Eur J Haematol. 2002. 69:227–235.
Article30. Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer. 2002. 87:555–561.
Article31. Pigault C, Follenius-Wund A, Schmutz M, Freyssinet JM, Brisson A. Formation of two-dimensional arrays of annexin V on phosphatidylserine-containing liposomes. J Mol Biol. 1994. 236:199–208.
Article32. Prakash P, Russell RM, Krinsky NI. In vitro inhibition of proliferation of estrogen-dependent and estrogen independent human breast cancer cells treated with carotenoids or retinoids. J Nutr. 2001. 131:1574–1580.
Article33. Ralhan R, Kaur J. Retinoids as chemopreventive agents. J Biol Regul Homeost Agents. 2003. 17:66–91.34. Raloff J. Antioxidants may help cancers thrive. Science News. 2000. 157:5.35. Rishi AK, Zhang L, Boyanapalli M, Wali A, Mohammad RM, Yu Y, Fontana JA, Hatfield JS, Dawson MI, Majumdar AP, Reichert U. Identification and characterization of a cell cycle and apoptosis regulatory protein-1 as a novel mediator of apoptosis signaling by retinoid CD437. J Biol Chem. 2003. 278:33422–33435.
Article36. Sato Y, Meller R, Yang T, Taki W, Simon RP. Stereo-selective neuroprotection against stroke with vitamin A derivatives. Brain Res. 2008. 1241:188–192.
Article37. Seewaldt VL, Johnson BS, Parker MB, Collins SJ, Swisshelm K. Expression of retinoic acid receptor beta mediates retinoic acid-induced growth arrest and apoptosis in breast cancer cells. Cell Growth Differ. 1995. 6:1077–1088.38. Smith MA, Parkinson DR, Cheson BD, Friedman MA. Retinoids in cancer therapy. J Clin Oncol. 1992. 10:839–864.
Article39. Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004. 24:201–221.
Article40. Stennicke HR, Salvesen GS. Caspase assays. Methods Enzymol. 2000. 322:91–100.
Article41. Sutherland MW, Learmonth BA. The tetrazolium dyes MTS and XTT provide new quantitative assays for superoxide and superoxide dismutase. Free Radic Res. 1997. 27:283–289.
Article42. Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxi Redox Signal. 2002. 4:405–414.
Article43. Villa P, Kaufmann SH, Earnshaw WC. Caspase and caspase inhibitors. Trends Biochem Sci. 1997. 22:388–393.44. Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol. 1982. 6:675–682.
Article45. Xu Q, Konta T, Kitamura M. Retinoic acid regulation of mesangial cell apoptosis. Exp Nephrol. 2002. 10:171–175.
Article46. Xu XC, Liu X, Tahara E, Lippman SM, Lotan R. Expression and up-regulation of retinoic acid receptor-beta is associated with retinoid sensitivity and colony formation in esophageal cancer cell lines. Cancer Res. 1999. 59:2477–2483.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Retinoids in Medulloblastoma Cell Culture
- Effects of alpha-lipoic acid on cell proliferation and apoptosis in MDA-MB-231 human breast cells
- Anti-proliferative efficacy comparison of conjugated linoleic acid on human cancer cell lines
- Apoptotic Effects of 6-Gingerol in Human Breast Cancer Cells
- Apoptosis and Cell Cycle Arrest in Two Human Breast Cancer Cell Lines by Dieckol Isolated from Ecklonia cava