Nutr Res Pract.
2007 Dec;1(4):273-278.
The inhibitory effect of natural bioactives on the growth of pathogenic bacteria
- Affiliations
-
- 1Department of Food and Nutritional Sciences, Ewha Womans University, Seoul 120-750, Korea. yhmoon@ewha.ac.kr
Abstract
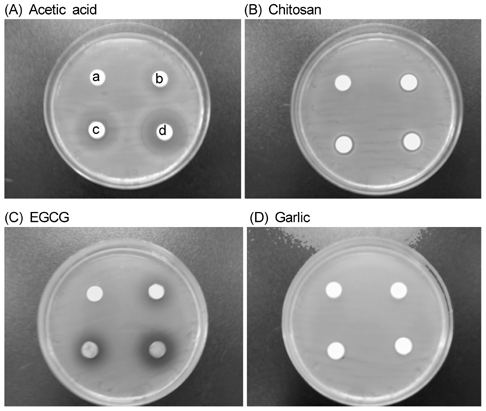
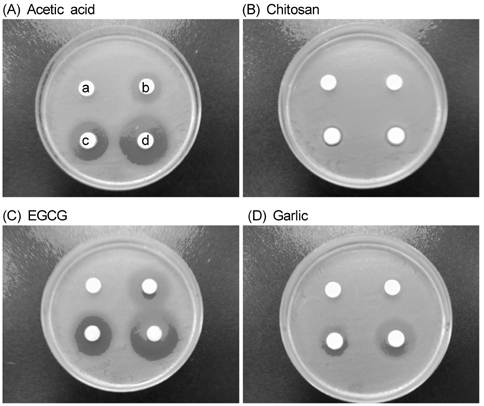
- The objective of this study was to evaluate the inhibitory activity of natural products, against growth of Escherichia coli (ATCC 25922) and Salmonella typhimurium (KCCM 11862). Chitosan, epigallocatechin gallate (EGCG), and garlic were used as natural bioactives for antibacterial activity. The testing method was carried out according to the disk diffusion method. All of chitosan, EGCG, and garlic showed inhibitory effect against the growth of E. coli and Salmonella typhi. To evaluate the antibacterial activity of natural products during storage, chicken skins were inoculated with 106 of E. coli or Salmonella typhi. The inoculated chicken skins, treated with 0.5, 1, or 2% natural bioactives, were stored during 8 day at 4degrees C. The numbers of microorganisms were measured at 8 day. Both chitosan and EGCG showed significant decrease in the number of E. coli and Salmonella typhi in dose dependent manner (P < 0.05). These results suggest that natural bioactives such as chitosan, EGCG may be possible to be used as antimicrobial agents for the improvement of food safety.
MeSH Terms
Figure
Reference
-
1. Bakri IM, Douglas CWI. Inhibitory effect of garlic extract on oral bacteria. Arch Oral Biol. 2005. 50:645–651.
Article2. Bin S, Yi ZC, John DB, Harold C. Antibacterial properties and major bioactive components of Cinnamon Stick (Cinnamomum burmannii): activity against food-borne pathogenic bacteria. J Agric Food Chem. 2007. 55:5484–5490.3. Cho MH, Bae EK, Ha SD, Park JY. Application of natural antimicrobials to food industry. Food Science and Industry. 2005. 38:36–45.4. Chung KT, Lu Z, Chou MW. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem Toxicol. 1998. 36:1053–1060.
Article5. Cooper R, Morre DJ, Morre J. Medicinal Benefits of Green Tea: Part I. Review of Noncancer Health Benefits. J Altern Complement Med. 2005. 11:521.
Article6. Daljit SA, Jasleen Kaur. Antimicrobial activity of spices. Int J Antimicrob Agents. 1999. 2:257–262.7. Davidsom PM, Parish ME. Method for testing the efficacy of food antimicrobials. Food Technology. 1989. 43:148–155.8. Dimitrios G, Ioannis A, Panagiota K, Georgios B, Spyridon AG. Effect of rosemary extract, chitosan and a-tocopherol on microbiological parameters and lipid oxidation of fresh pork sausages stored at 4℃. Meat Sci. 2007. 76:172–181.9. Elena DR, Rosa C, Miguel P, Carlos AC. Comparison of pathogenic and spoilage bacterial levels on refrigerated poultry parts following treatment with trisodium phosphate. Food Microbiol. 2006. 23:195–198.
Article10. Elena DR, Monica PM, Miguel P, Carlos AC, Rosa C. Effect of various chemical decontamination treatments on natural microflora and sensory characteristics of poultry. Int J Food Microbiol. 2007. 115:268–280.
Article11. Entsar IR, Mohamed ET, Christian VS, Guy S, Walter S. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules. 2003. 6:1457–1465.
Article12. Fernandez LJ, Zhi N, Aleson CL, Perez JA, Kuri V. Antioxidant and antibacterial activities of natural extracts: application in beef meatballs. Meat Sci. 2005. 69:371–380.
Article13. Hamilton-Miller JM. Antimicrobial properties of tea (Camellia sinensis L.). Antimicrob Agents Chemother. 1995. 39:2375–2377.
Article14. Kong YJ, Park BK, Oh DH. Antimicrobial activity of Quercus mongolica leaf ethanol extracts and organic acids against food-borne microorganisms. Korean Journal of Food Science and Technology. 2001. 33:178–183.15. Mau JL, Chen CP, Hsieh PC. Antimicrobial effect of extracts from Chinese Chive, Cinnamon, and Corni Fructus. J Agric Food Chem. 2001. 49:183–188.
Article16. No HK, Park NY, Lee SH, Hwang HJ, Meyers SP. Antibacterial activities of chitosans and chitosan oligemers with different molecular weights on Spoilage Bacteria isolated from Tofu. J Food Sci. 2002. 67:1511–1514.
Article17. Olasupo NA, Fitzgerald DJ, Gasson MJ, Narbad A. Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar Tuphimurium. Lett Appl Microbiol. 36:448–451.18. Patsias A, Chouliara I, Badeka A, Savvaidis IN, Kontominas MG. Shelf-life of a chilled precooked chicken product stored in air and under modified atmospheres: microbiological, chemical, sensory attributes. Food Microbiol. 2006. 23:423–429.
Article19. Ross ZM, O'gara EA, Hill DJ, Sleightholme H.V, Maslin DJ. Antimicrobial properties of Garlic oil against human enteric bacteria: Evaluation of methodologies and comparisons with Garlic oil sulfides and Garlic powder. Appl Environ Microbiol. 2001. 67:475–480.
Article20. Shin JH, Lee SY, Dougherty RH, Rasco B, Kang DH. Combined effect of mild heat and acetic acid treatment for inactivating Escherichia coli 0157:H7, Listeria monocytogenes and Salmonella typhimurium in an asparagus puree. J Appl Microbiol. 2006. 101:1140–1151.
Article21. Sibel R. Natural antimicribials for the minimal processing of foods. Camb Munic Code 1988 Camb Mass. 2003. 1–295.22. Sallam KhI, Ishioroshi M, Samejima K. Antioxidant and antimicrobial effects of garlic in chicken sausage. Lebenson Wiss Technol. 2004. 37:849–855.
Article23. Sallam KI, Samejima K. Effects of Trisosdium phosphate and Sodium chloride dipping on the microbial quality and shelf life of refrigerated tray-packaged chicken breasts. Food Sci Biotechnol. 2004. 13:425–438.24. Sallam KI, Samejima K. Microbiological and chemical quality of ground beef treated with sodium lactate and sodium chloride during refrigerated storage. Lebenson Wiss Technol. 2004. 37:865–892.
Article25. Sami F, Pierluigi C, Valentina C, Carlo IG, Tuberoso CI, Alberto A, Sandro D, Nejib M, Paolo C. Antimicrobial activity of Tunisian Quince (Cydonia oblonga miller) pulp and peel polyphenolic extracts. J Agric Food Chem. 2007. 55:963–969.
Article26. Serge A, David M. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999. 125–129.27. Weiduo SI, Joshua G, Rong T, Milosh K, Raymond Y, Yulong Y. Bioassay-guided purification and identification of antimicrobial components in Chinese green tea extract. J Chromatogr A. 2006. 1125:204–210.
Article28. Wang Y, Zhou P, Yu J, Pan X, Wang P, Lan W, Tao S. Antimicrobial effect of Chitooligosaccharides produced by Chitosanase from Pseudomonas CUY8. Asia Pac J Clin Nutr. 2007. 16:174–177.29. Yin MC, Cheng WS. Antioxidant and antimicrobial effects of four garlic-derived organofulfur compounds in ground beef. Meat Sci. 2003. 63:23–28.
Article30. Yoshida H, Katsuzaki H, Ohta R, Ishikawa K, Fukuda H, Fujino T, Suzuki A. An organosulfur compound isolated from oil-macerated garlic extract, and its antimicrobial effect. Biosci Biotechnol Biochem. 1999. 63:588–590.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study on the effect of sterilization of the thermometer c three disinfectant sponges
- Antibacterial and Antifungal Activities of Stereum ostrea, an Inedible Wild Mushroom
- Antimicrobial activity of essential oil of Eucalyptus globulus against fish pathogenic bacteria
- Anti-Bacterial Effect of Lactobacillus rhamnosus Cell-Free Supernatant Possessing Lysozyme Activity Against Pathogenic Bacteria
- Antifungal Activity of Lactic Acid Bacteria Strains Isolated from Natural Honey against Pathogenic Candida Species