Nutr Res Pract.
2007 Jun;1(2):94-99.
Effect of mucilage from yam on activation of lymphocytic immune cells
- Affiliations
-
- 1School of Bioresource Science, Andong National University, Andong, Gyeongbuk 760-749, Korea. okjhlee@andong.ac.kr
- 2Department of Genetic Engineering, Sungkyunkwan University, Suwon, Gyeonggi 440-746, Korea.
Abstract
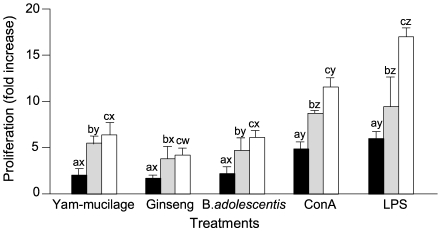
- The immunostimulating activities of mucilage fraction from yam were investigated. The proliferation of BSA-primed lymph node cells was enhanced between 4.1- to 10.9-fold compare to control, when cultured with 1 to 25 microgram/mL of yam-mucilage fraction. It showed strong immunopotentiating activity than ginseng extract and as remarkable as Bifidobacterium adolescentis M101-4 known as a positive immunostimulator. Mitogenicity to lymph node cells was fully induced by concanavalin A and lipopolysaccharide. The proliferation of splenocytes and Peyer's patch cells was enhanced between 5.0- to 14.1-fold and 2.4- to 6.4-fold, respectively, when cultured with 1 to 25 microgram/mL of yam-mucilage fraction. It enhanced the production of cytokines such as tumor necrosis factor-alpha and IL-6 in the culture of RAW 264.7 macrophage cells. In the culture of lipopolysaccharide-stimulated RAW 264.7 cells, production of cytokines was as similar as compared to controls. In unstimulated RAW 264.7 cells, both tumor necrosis factor-alpha and IL-6 production were enhanced between 15.6- to 60.1-fold and 2.3- to 9.1-fold, respectively. Mucilage fraction from yam is expected to be a safe immunopotentiator to maintain the host immunity and develop a physiologically functional food.
MeSH Terms
Figure
Reference
-
1. Chihara G, Hamuro J, Maeda YY, Shiio T, Suga T, Takasuka N, Sasaki T. Antitumor and metastasis-inhibitory activities of lentinan as an immunomodulator: an overview. Cancer Detect Prev Suppl. 1987; 1:423–443. PMID: 3319150.2. Choi IS, Lee IS, Ku SJ. The study on rheological and thermal properties of Dioscorea batatas DECAISNE starch. Korean Journal of Food and Cookery Science. 1992; 8:57–63.3. Damais C, Bona C, Chedid L, Fleck J, Nauciel C, Martin JP. Mitogenic effect of bacterial peptidoglycans possessing adjuvant activity. J Immunol. 1975; 115:268–271. PMID: 807626.4. Dong W, Azcona-Olivera JI, Brooks KH, Linz JE, Pestka JJ. Elevated gene expression and production of interleukins 2, 4, 5, and 6 during exposure to vomitoxin (deoxynivalenol) and cycloheximide in the EL-4 thymoma. Toxicol Appl Pharmacol. 1994; 127:282–290. PMID: 8048072.
Article5. Ha YD, Lee SB, Kwak YG. Removal of heavy metal and ACE inhibition of yam mucilage. Journal of the Korean Society of Food Science and Nutrition. 1998; 27:751–755.6. Han YN, Han SH, Lee IR. Purification of mucilages from Dioscorea batatas and D. japonica and their content analysis. Korean Journal of Pharmacognosy. 1990; 21:274–283.7. Jung SK, Jung YY, Jung WS. Studies on the browning inhibition of yam (Dioscorea aimadoimo) during hot air dehydration. Journal of the Korean Society of Agricultural Chemistry and Biotechnology. 1996; 39:384–388.8. Kim HS, Kim SS, Park YG, Seog HM. Physicochemical properties of several Korean yam starches. Korean Journal of Food Science and Technology. 1991; 23:554–560.9. Kim YS, Lee BE, Kim KJ, Lee YT, Cho BK, Chung YC. Antitumor and immunomodulatory activities of the P. grandiflorum cultivated for more than 20 years. Yakhak Hoeji. 1998; 42:382–387.10. Kwon EG, Choe EM, Gu SJ. Effects of mucilage from Yam (Dioscorea batatas DECENE) on blood glucose and lipid composition in alloxan-induced diabetic mice. Korean Journal of Food Science and Technology. 2001a; 33:795–801.11. Kwon J, Lee SJ, So JN, Oh CH. Effects of Schizandra chinensis fructus on the immunoregulatory action and apoptosis of L1210 cells. Korean Journal of Food Science and Technology. 2001b; 33:384–388.12. Kwon JH, Lee KD, Lee SJ, Jung SK, Choi JW. Changes in chemical components and physical properties with freeze drying and hot air-drying of Dioscorea batatas. Journal of the Korean Society of Food Science and Nutrition. 1998; 27:908–913.13. Lee BY, Kim HG. Quality properties of korean yam by various drying methods. Korean Journal of Food Science and Technology. 1998; 30:877–882.14. Lee BY, Lee YC, Kim HM, Kim CJ, Park MH. Rheological properties of the gelatinized yam starch solution. Korean Journal of Food Science and Technology. 1992; 24:619–622.15. Lee BY, Park DJ, Ku KH, Kim HG, Mok CK. Mucilage separation of Korean yam using microparticulation/air classification process. Korean Journal of Food Science and Technology. 1994; 26:596–602.16. Lee CB. Illustrated of Flora of Korea. 1982. Seoul. Republic of Korea: Hyung-Moon Publishing Co.;p. 225.17. Lee HY, Lee HS. Stimulatory effect of Korean red-ginseng extract on the proliferation and cellular activity of lymphocytes. Journal of Ginseng Research. 1998; 22:60–65.18. Lee IM. Characteristics and functionalities of biologically active substances from yam (dioscorea batatas DECENE). 1996. Kyung Hee University.19. Lee J, Ametani A, Enomoto A, Sato Y, Motoshima H, Ike F, Kaminogawa S. Screening for the immunopotentiation activity of food microorganisms and enhancement of the immune response by Bifidobacterium adolescentis M101-4. Biosci Biotechnol Biochem. 1993; 57:2127–2132.20. Lim SD, Seong KS, Kim KS, Han DU. Effects of fermented milk containing herb extract from Acanthopanax divaricatus var. albeofructus and Codonopsis Ianceolata on the immune status of mouse. Korean Journal for Food Science of Animal Products. 2007; 27:95–101.
Article21. Medicine Botany Society for the Research. New Medicine Botany. 1993. Seoul. Republic of Korea: Hak-Chang Publishing Co.;p. 228–229.22. Misaki A, Ito T, Harada T. Constitutional studies on the mucilage of "Yamanoimo" Dioscorea batatas Decne, forma Tsukune. Agricultural and Biological Chemistry. 1972; 36:761–771.23. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 16:55–63. PMID: 6606682.
Article24. Ohtani K, Murakami K. Structure of mannan fractionated from water-soluble mucilage Nagaimo (Dioscorea batatas DECNE). Agricultural and Biological Chemistry. 1991; 55:2413–2414.25. Sekine K, Watanabe-Sekine E, Toida T, Kawashima T, Kataoka T, Hashimoto Y. Adjuvant activity of the cell wall of Bifidobacterium infantis for in vivo immune responses in mice. Immunopharmacol Immunotoxicol. 1994; 16:589–609. PMID: 7876463.26. Sminia T, Wilders MM, Janes EM, Hoefsmit EC. Characteristics of non-lymphoid cells in Peyer's patches of the rat. Immunobiology. 1983; 164:136–143. PMID: 6574104.27. Suzuki I, Hashimoto K, Yadomae T. Rapid preparation of functional murine Peyer's patch cells. Bioscience Microflora. 1990; 9:87–98.
Article28. Tanoue H, Simozono H. Chemical and rheological properties of viscous polysaccharides from three species of yam (Dioscorea). Nippon Shokuhin Kyogo Gakkaishi. 1991; 38:751–757.29. Tomoda M, Ishikawa K, Yokoi M. Plant mucilages. XXX. isolation and characterization of a mucilage, "Dioscorea-mucilage B" from the rhizophors of Dioscorea batatas. Chem Pharm Bull. 1981; 29:3256–3261.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of a thermal stable allergen in yam (Dioscorea opposita) to cause anaphylaxis
- Extrinsic Acquisition of CD80 by Antigen-Specific CD8⺠T Cells Regulates Their Recall Immune Responses to Acute Viral Infection
- Leaf Spot of Yam Caused by Pseudophloeosporella dioscoreae in Korea
- Metabolic Reprogramming by the Excessive AMPK Activation Exacerbates Antigen-Specific Memory CD8⺠T Cell Differentiation after Acute Lymphocytic Choriomeningitis Virus Infection
- Post-transcriptional Regulation of NK Cell Activation