Nat Prod Sci.
2016 Mar;22(1):13-19. 10.20307/nps.2016.22.1.13.
Anti-osteoporotic and Antioxidant Activities by Rhizomes of Kaempferia parviflora Wall. ex Baker
- Affiliations
-
- 1College of Pharmacy, Chungnam National University, Daejeon 305-764, Republic of Korea. yhk@cnu.ac.kr
- 2Institute of Marine Biochemistry (IMBC), Vietnam Academy of Science and Technology (VAST), 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam.
- 3Department of Food and Nutrition, Hannam University, Daejeon 305-811, Republic of Korea. haedong@hnu.kr
- KMID: 2312915
- DOI: http://doi.org/10.20307/nps.2016.22.1.13
Abstract
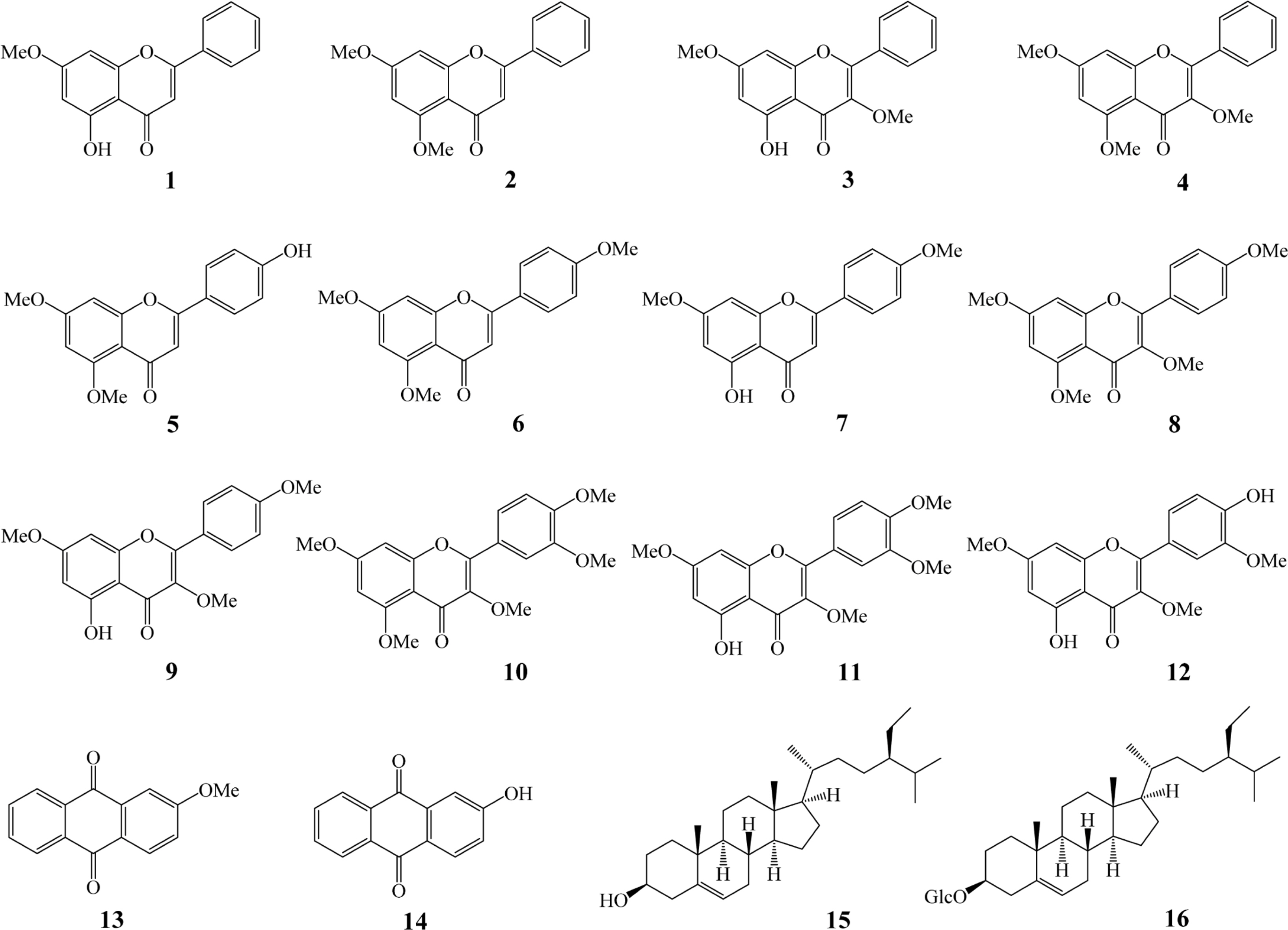
- In this report, we investigated the antioxidant (peroxyl radical-scavenging and reducing capacities) and anti-osteoporotic activities of extracts and isolated constituents (1 - 16) from the rhizomes of Kaempferia parviflora Wall. ex Baker on pre-osteoclastic RAW 264.7 cells. Compound 5 exhibited significant peroxyl radical-scavenging capacity, with TE value of 8.47 ± 0.52 µM, while compound 13 showed significant reducing capacity, with CUPRAC value of 5.66 ± 0.26 µM, at 10.0 µM. In addition, flavonoid compounds 2, 4, 6, 8, 10, 12, and terpene compound 15 showed significant inhibition of tartrate-resistant acid phosphatase (TRAP) in NF-κB ligand-induced osteoclastic RAW 264.7 cells, with values ranging from 16.97 ± 1.02 to 64.67 ± 2.76%. These results indicated that K. parviflora could be excellent sources for the antioxidant and anti-osteoporotic traditional medicinal plants.
Figure
Reference
-
(1). Hu F. B., Willett W. C. J.Am. Med. Assoc. 2002; 288:2569–2578.(2). Pietta P. G. J.Nat. Prod. 2000; 63:1035–1042.(3). Kong Y. Y., Yoshida H., Sarosi I., Tan H. L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A. J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C. R., Lacey D. L., Mak T. W., Boyle W. J., Penninger J. M.Nature. 1999; 397:315–323.(4). Yenjai C., Prasanphen K., Daodeeb S., Wongpanich V., Kittakoop P.Fitoterapia. 2004; 75:89–92.(5). Sutthanut K., Sripanidkulchai B., Yenjai C., Jay M. J.Chromatogr. A. 2007; 1143:227–233.(6). Chaipech S., Morikawa T., Ninomiya K., Yoshikawa M., Pongpiriyadacha Y., Hayakawa T., Muraoka O.Chem. Pharm. Bull. 2012; 60:62–69.(7). Azuma T., Kayano S. I., Matsumura Y., Konishi Y., Tanaka Y., Kikuzaki H.Food Chem. 2011; 125:471–475.(8). Sawasdee P., Sabphon C., Sitthiwongwanit D., Kokpol U.Phytother. Res. 2009; 23:1792–1794.(9). Patanasethanont D., Nagai J., Yumoto R., Murakami T., Sutthanut K., Sripanidkulchai B. O., Yenjai C., Takano M. J.Pharma. Sci. 2007; 96:223–233.(10). Wattanapitayakul S. K., Suwatronnakorn M., Chularojmontri L., Herunsalee A., Niumsakul S., Charuchongkolwongse S., Chansuvanich N. J.Ethnopharmacol. 2007; 110:559–562.(11). Akase T., Shimada T., Terabayashi S., Ikeya Y., Sanada H., Aburada M. J.Nat. Med. 2011; 65:73–80.(12). Wong C. S., Tansakul P., Tewtrakul S. J.Ethnopharmacol. 2009; 124:576–580.(13). Tewtrakul S., Subhadhirasakul S., Kummee S. J.Ethnopharmacol. 2008; 116:191–193.(14). Wattanapitayakul S. K., Chularojmontri L., Herunsalee A., Charuchongkolwongse S., Chansuvanich N.Fitoterapia. 2008; 79:214–216.(15). Patanasethanont D., Nagai J., Matsuura C., Fukui K., Sutthanut K., Sripanidkulchai B.O., Yumoto R., Takano M.Eur. J. Pharmacol. 2007; 566:67–74.(16). Yenjai C., Wanich S.Bioorg. Med. Chem. Lett. 2010; 20:2821–2823.(17). Aruoma O. I., Deiana M., Jenner A., Halliwell B., Kaur H., Banni S., Corongiu F. P., Dessí M. A., Aeschbach R. J.Agric. Food Chem. 1998; 46:5181–5187.(18). Kurihara H., Fukami H., Asami S., Toyoda Y., Nakai M., Shibata H., Yao X. S.Biol. Pharm. Bull. 2004; 27:1093–1098.(19). van de Loosdrecht A. A., Beelen R. H. J., Ossenkoppele G. J., Broekhoven M. G., Langenhuijsen M. M. A.C. J. Immunol. Methods. 1994; 174:311–320.(20). Lee S. H., Ding Y., Yan X. T., Kim Y. H., Jang H. D. J.Nat. Prod. 2013; 76:615–620.(21). Vichitphan S., Vichitphan K., Sirikhansaeng P.KMITL Sci. Tech. J. 2007; 7:97–105.(22). Horikawa T., Shimada T., Okabe Y., Kinoshita K., Koyama K., Miyamoto K. I., Ichinose K., Takahashi K., Aburada M.Biol. Pharm. Bull. 2012; 35:686–692.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phytochemical Constituents from the Rhizomes of Osmunda japonica Thunb and Their Anti-oxidant Activity
- Determination of the Factors Influencing Rupture of Baker's Cysts in the Knee on Plain Radiographs and MRI
- Baker's Cyst Filled with Hematoma at the Lower Calf
- In vivo antioxidant, hypoglycemic, and anti-tumor activities of anthocyanin extracts from purple sweet potato
- Fermented Product Extract with Lentinus edodes Attenuate the Inflammatory Mediators Releases and Free Radical Production