Nat Prod Sci.
2015 Dec;21(4):293-296. 10.20307/nps.2015.21.4.293.
Epi-Leptosphaerin: A New L-Isoascorbic Acid Derivative from Marine Sponges
- Affiliations
-
- 1College of Pharmacy, Chungnam National University, Daejeon 305-764, Korea. mkna@cnu.ac.kr
- KMID: 2312913
- DOI: http://doi.org/10.20307/nps.2015.21.4.293
Abstract
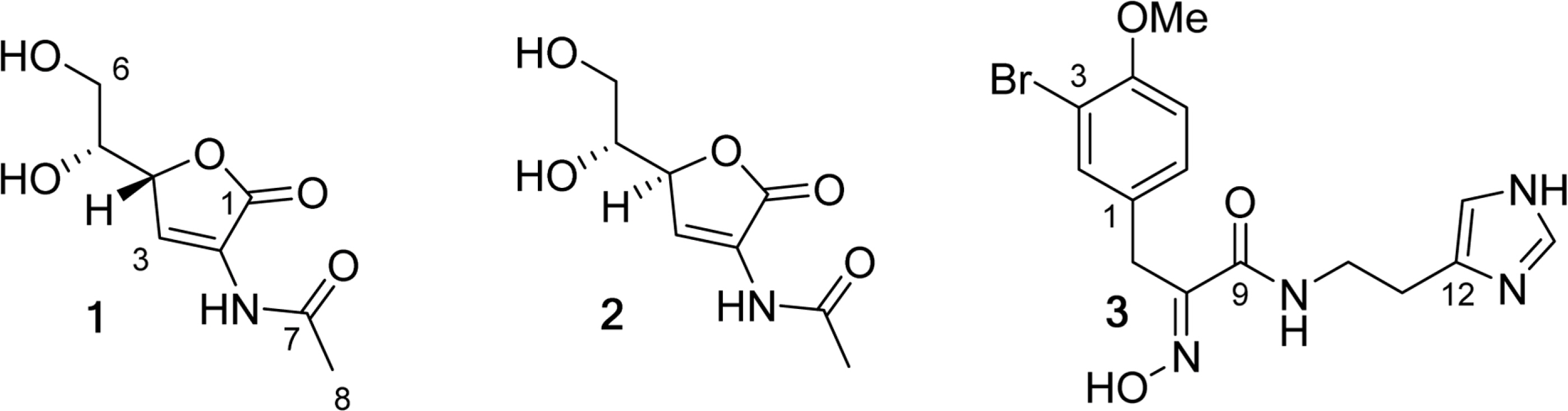
- A new L-isoascorbic acid derivative epi-leptosphaerin (1) and two known compounds leptosphaerin (2), and verongamine (3) were isolated from sponges of the orders Verongida and Thorectidae. Compounds 1 and 2 are most likely of sponge-associated fungal origin. In the present study, isolated compounds were investigated for their inhibition of soluble epoxide hydrolase (sEH), which is considered a promising target for the management of pain, inflammation, and comorbidities associated with diabetes. Compound 3, verongamine, displayed weak inhibitory activity against sEH with an IC50 value 51.5 +/- 1.0 microM.
Figure
Reference
-
(1). Perdicaris S., Vlachogianni T., Valavanidis A.Nat. Prod. Chem. Res. 2013; 1:114.(2). Simmons T. L., Coates R. C., Clark B. R., Engene N., Gonzalez D., Esquenazi E., Dorrestein P. C., Gerwick W. H.Proc. Natl. Acad. Sci. 2008; 105:4587–4594.(3). Abdelmohsen U. R., Bayer K., Hentschel U.Nat. Prod. Rep. 2014; 31:381–399.(4). Suryanarayanan T. S.Bot. Mar. 2012; 55:553–564.(5). Lemmens-Gruber R., Kamyar M. R., Dornetshuber R.Curr. Med. Chem. 2009; 16:1122–1137.(6). Imig J. D., Hammock B. D.Nat. Rev. Drug Discov. 2009; 8:794–805.(7). Hammock B. D., Wagner K., Inceoglu B.Pain Manag. 2011; 1:383–386.(8). Kochanowska A. J., Rao K. V., Childress S., El-Alfy A., Matsumoto R. R., Kelly M., Stewart G. S., Sufka K. J., Hamann M. T. J.Nat. Prod. 2008; 71:186–189.(9). Hwang I. H., Oh J., Kochanowska-Karamyan A., Doerksen R. J., Na M., Hamann M. T.Tetrahedron Lett. 2013; 54:3872–3876.(10). Kulkarni R., Kim J. H., Kim Y. H., Oh S., Na M.Nat. Prod. Sci. 2015; 21:25–29.(11). Hwang I. H., Oh J., Zhou W., Park S., Kim J.-H., Chittiboyina A. G., Ferreira D., Song G. Y., Oh S., Na M.Nat. Prod. 2015; 78:453–461.(12). White J. D., Badger R. A., Kezar III H. S., Pallenberg A. J., Schiehser G. A.Tetrahedron. 1989; 45:6631–6644.(13). Miroslav P., Emmanuel Z., Fletcher H. G. Jr.Carbohydr. Res. 1975; 43:345–354.(14). Hanisak M. D., Samuel M. A.Dev. Hydrobiol. 1987; 41:399–404.(15). Takahashi C., Numata A., Ito Y., Matsumura E., Araki H., Iwaki H., Kushida K. J.Chem. Soc. Perkin Trans. 1994; 1:1859–1864.(16). Kyotani D., Hasegawa K., Ohishi H., Wu W., Wang L., Hasumi K.Biosci. Biotechnol. Biochem. 2009; 73:954–956.(17). Mierzwa R., King A., Conover M. A., Tozzi S., Puar M. S., Patel M., Coval S. J., Pomponi S. A. J.Nat. Prod. 1994; 57:175–177.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Marine Sponges as a Drug Treasure
- Violapyrone J, alpha-Pyrone Derivative from a Marine-derived Actinomycetes, Streptomyces sp.
- Calyxaprenols A-D, New Merohexaprenoid Metabolites from the Marine Sponge Calyx sp.
- A New Record of Penicillium antarcticum from Marine Environments in Korea
- Effect of epidural analgesia on cesarean section in nulliparous women