Nat Prod Sci.
2015 Dec;21(4):282-288. 10.20307/nps.2015.21.4.282.
Viriditoxin Induces G2/M Cell Cycle Arrest and Apoptosis in A549 Human Lung Cancer Cells
- Affiliations
-
- 1College of Pharmacy, Pusan National University, Busan 609-735, Korea. jhjung@pusan.ac.kr
- KMID: 2312911
- DOI: http://doi.org/10.20307/nps.2015.21.4.282
Abstract
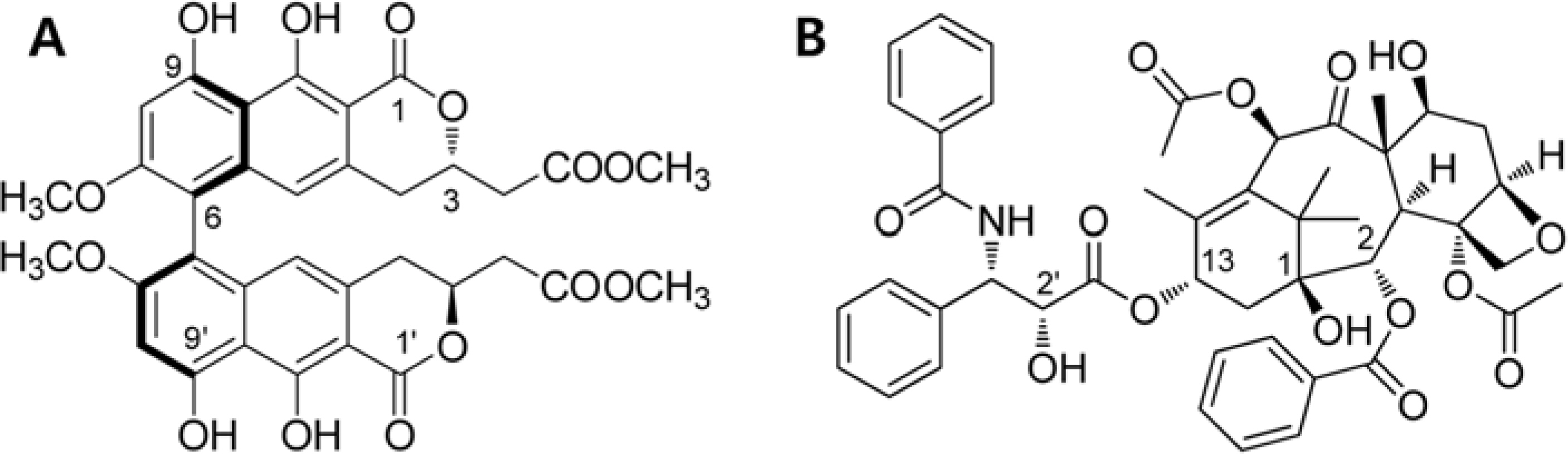
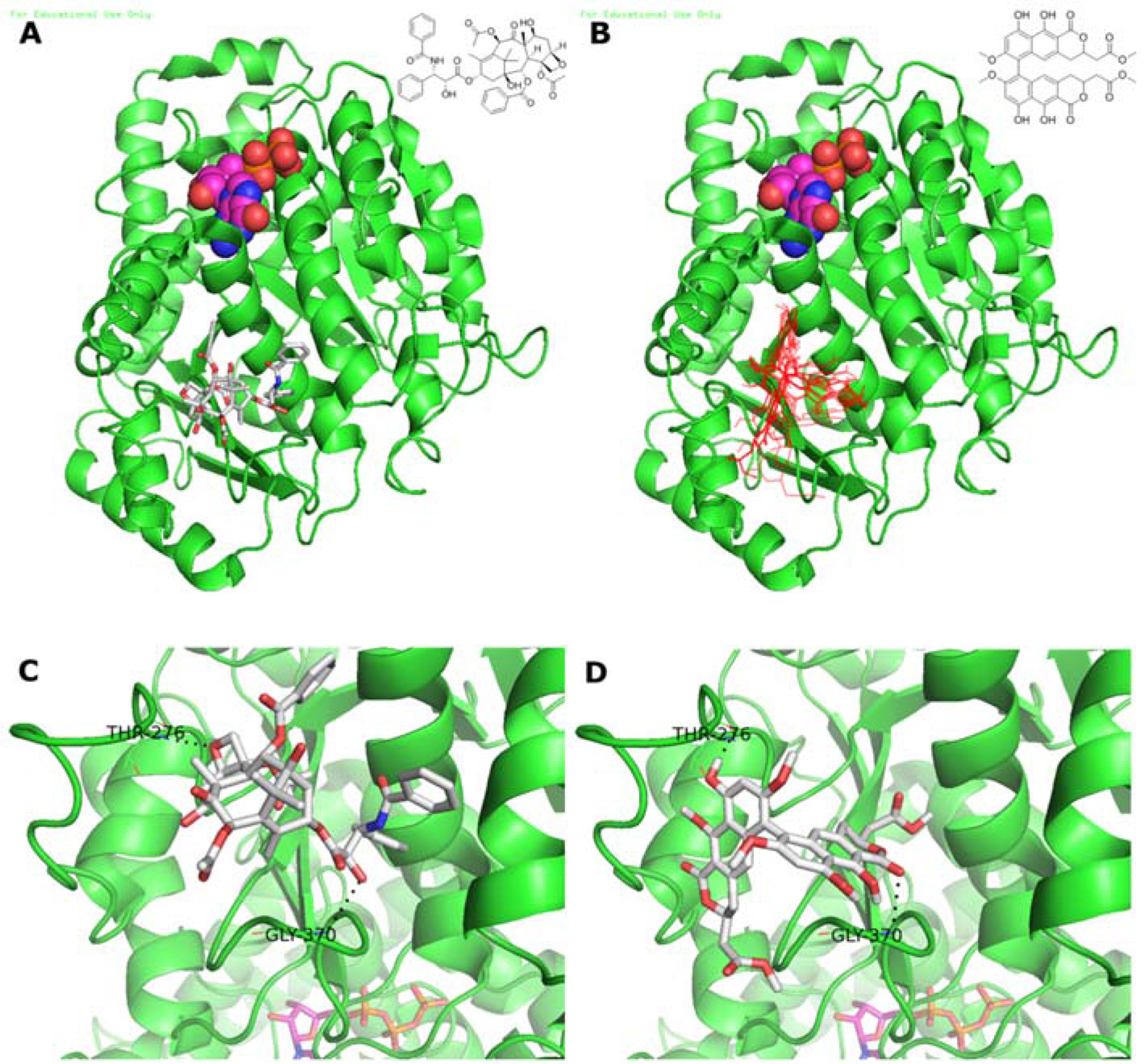
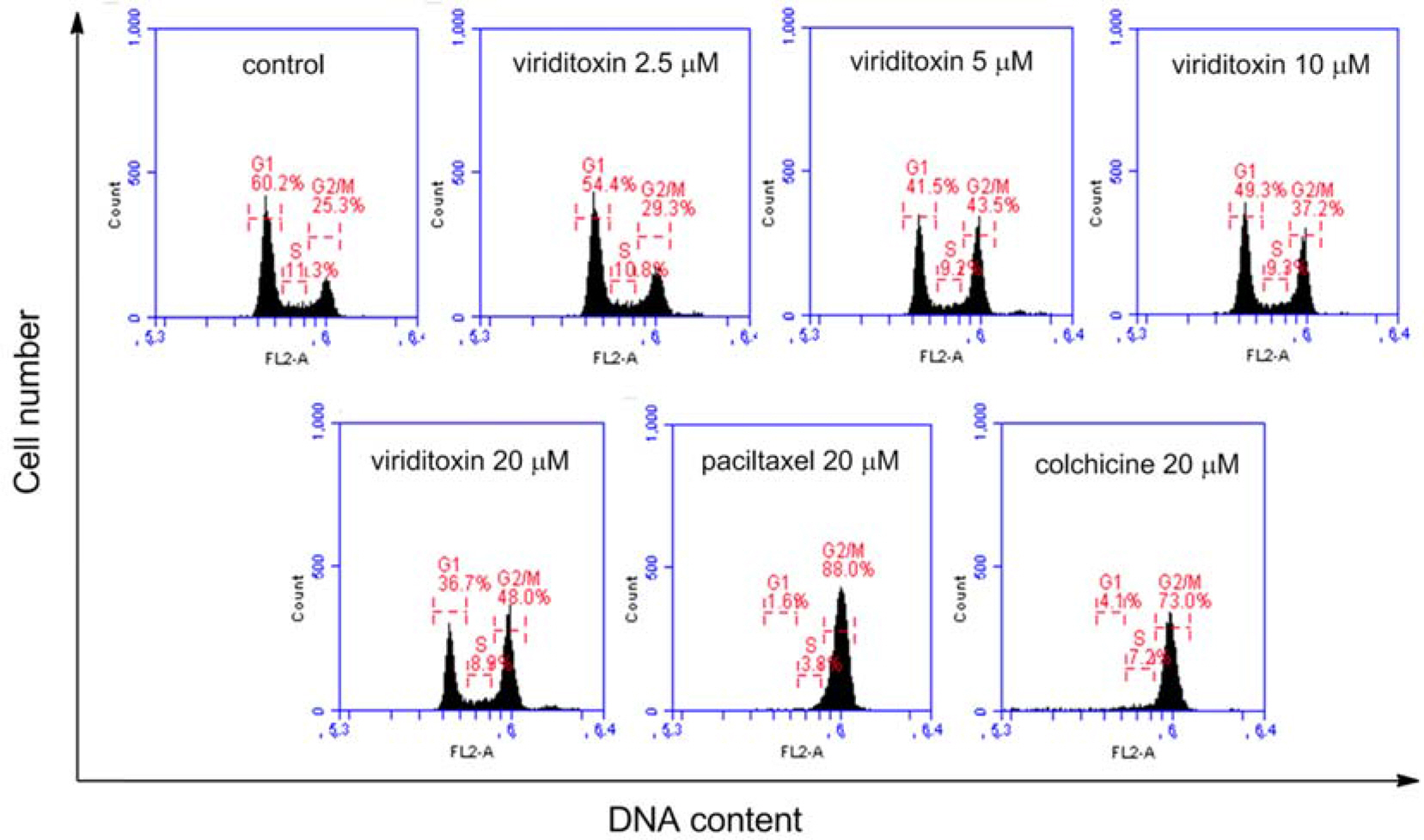
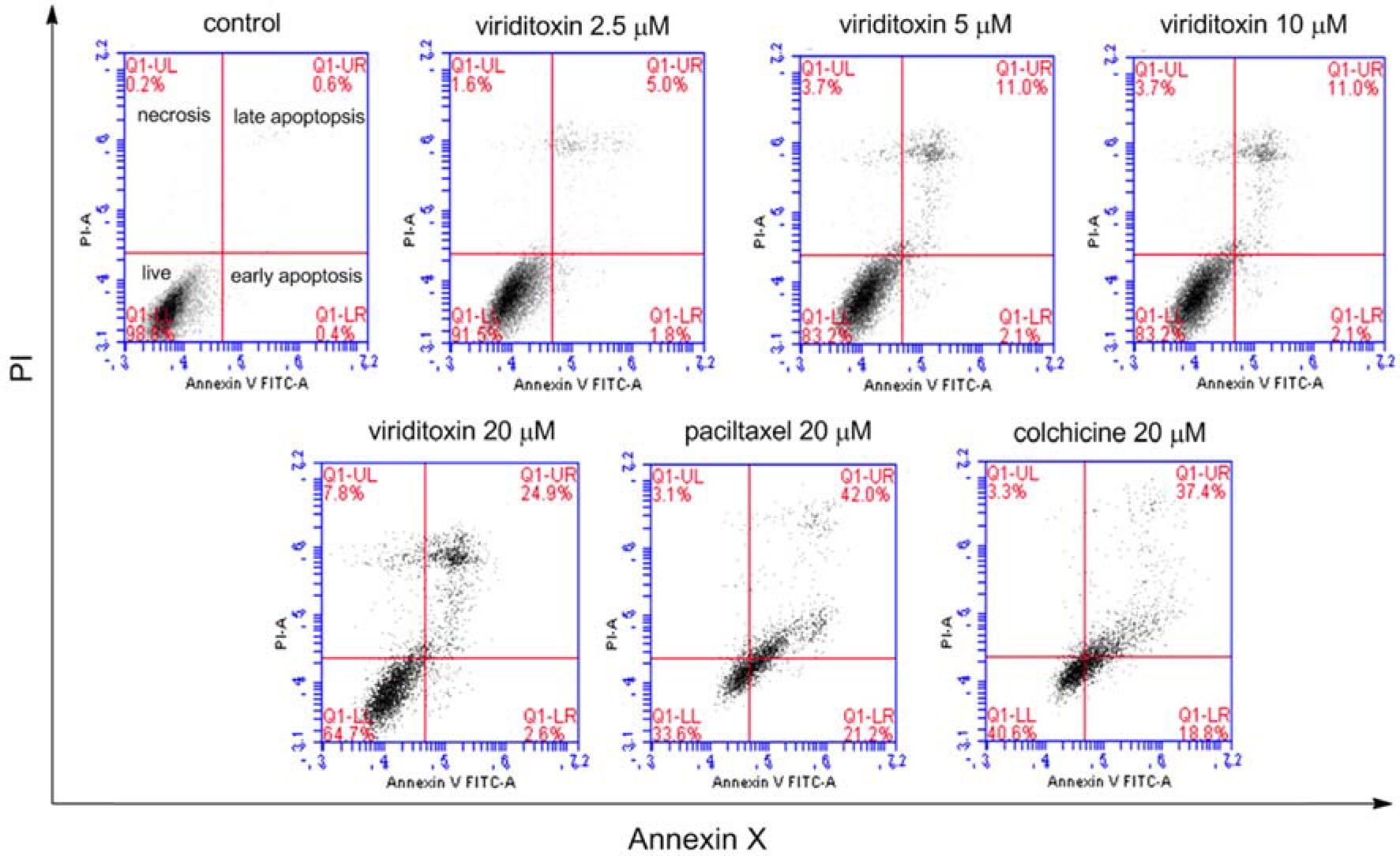
- Viriditoxin is a fungal metabolite isolated from Paecilomyces variotii, which was derived from the giant jellyfish Nemopilema nomurai. Viriditoxin was reported to inhibit polymerization of FtsZ, which is a key protein for bacterial cell division and a structural homologue of eukaryotic tubulin. Both tubulin and FtsZ contain a GTP-binding domain, have GTPase activity, assemble into protofilaments, two-dimensional sheets, and protofilament rings, and share substantial structural identities. Accordingly, we hypothesized that viriditoxin may inhibit eukaryotic cell division by inhibiting tubulin polymerization as in the case of bacterial FtsZ inhibition. Docking simulation of viriditoxin to beta-tubulin indicated that it binds to the paclitaxel-binding domain and makes hydrogen bonds with Thr276 and Gly370 in the same manner as paclitaxel. Viriditoxin suppressed growth of A549 human lung cancer cells, and inhibited cell division with G2/M cell cycle arrest, leading to apoptotic cell death.
Keyword
MeSH Terms
Figure
Reference
-
(1). Liu J., Li F., Kim E. L., Hong J. K., Jung J. H.Nat. Prod. Sci. 2013; 19:61–65.(2). Wang J., Galgoci A., Kodali S., Herath K. B., Jayasuriya H., Dorso K., Vicente F., González A., Cully D., Bramhill D., Singh S. J.Biol. Chem. 2003; 278:44424–44428.(3). Wong D. T., Hamill R. L.Biochem. Biophys. Res. Commun. 1976; 71:332–338.(4). Löwe J., Amos L. A.Nature. 1998; 391:203–206.(5). Tian W., Xu D., Deng Y. C.Pharmazie. 2012; 67:811–816.(6). Erickson H. P.Cell. 1995; 80:367–370.(7). Bi E. F., Lutkenhaus J.Nature. 1991; 354:161–164.(8). Chen Y., Erickson H. P. J.Biol. Chem. 2005; 280:22549–22554.(9). Popp D., Iwasa M., Erickson H. P., Narita A., Maéda Y., Robinson R. C. J.Biol. Chem. 2010; 285:11281–11289.(10). Anderson D. E., Kim M. B., Moore J. T., O'Brien T. E., Sorto N. A., Grove C. I., Lackner L. L., Ames J. B., Shaw J. T.ACS Chem. Biol. 2012; 7:1918–1928.(11). Läppchen T., Pinas V. A., Hartog A. F., Koomen G. J., Schaffner-Barbero C., Andreu J. M., Trambaiolo D., Löwe J., Juhem A., Popov A. V., den Blaauwen T.Chem. Biol. 2008; 15:189–199.(12). Foss M. H., Eun Y. J., Grove C. I., Pauw D. A., Sorto N. A., Rensvold J. W., Pagliarini D. J., Shaw J. T., Weibel D. B.Med. Chem. Commun. 2013; 4:112–119.(13). Gupta K. K., Bharne S. S., Rathinasamy K., Naik N. R., Panda D.FEBS J. 2006; 273:5320–5332.(14). Rai D., Singh J. K., Roy N., Panda D.Biochem. J. 2008; 410:147–155.(15). Wang J., Galgoci A., Kodali S., Herath K. B., Jayasuriya H., Dorso K., Vicente F., González A., Cully D., Bramhill D., Singh S. J.Biol. Chem. 2003; 278:44424–44428.(16). Hsiao C. J., Hsiao G., Chen W. L., Wang S. W., Chiang C. P., Liu L. Y., Guh J. H., Lee T. H., Chung C. L. J.Nat. Prod. 2014; 77:758–765.(17). Zhu Z., Sun H., Ma G., Wang Z., Li E., Liu Y., Liu Y.Int. J. Mol. Sci. 2012; 13:2025–2035.(18). Kundu S., Kim T. H., Yoon J. H., Shin H. S., Lee J., Jung J. H., Kim H. S.Int. J. Oncol. 2014; 45:2331–2340.(19). Löwe J., Li H., Downing K. H., Nogales E. J.Mol. Biol. 2001; 313:1045–1057.(20). Sun L., Simmerling C., Ojima I.ChemMedChem. 2009; 4:719–731.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiproliferative Activity of Gibbosic Acid H through Induction of G0/G1 Cell Cycle Arrest and Apoptosis in Human Lung Cancer Cells
- Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells
- Sesamin induces A549 cell mitophagy and mitochondrial apoptosis via a reactive oxygen species-mediated reduction in mitochondrial membrane potential
- The Effect of Irradiation and Epidermal Growth Factor on Cell Cycle and Apoptosis Induction in Human Epithelial Tumor Cell Lines
- Knockdown of the M2 Isoform of Pyruvate Kinase (PKM2) with shRNA Enhances the Effect of Docetaxel in Human NSCLC Cell Lines In Vitro