Nat Prod Sci.
2015 Dec;21(4):273-277. 10.20307/nps.2015.21.4.273.
Salternamide E from a Saltern-derived Marine Actinomycete Streptomyces sp.
- Affiliations
-
- 1Natural Products Research Institute, College of Pharmacy, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 151-742, Republic of Korea. dongchanoh@snu.ac.kr
- KMID: 2312909
- DOI: http://doi.org/10.20307/nps.2015.21.4.273
Abstract
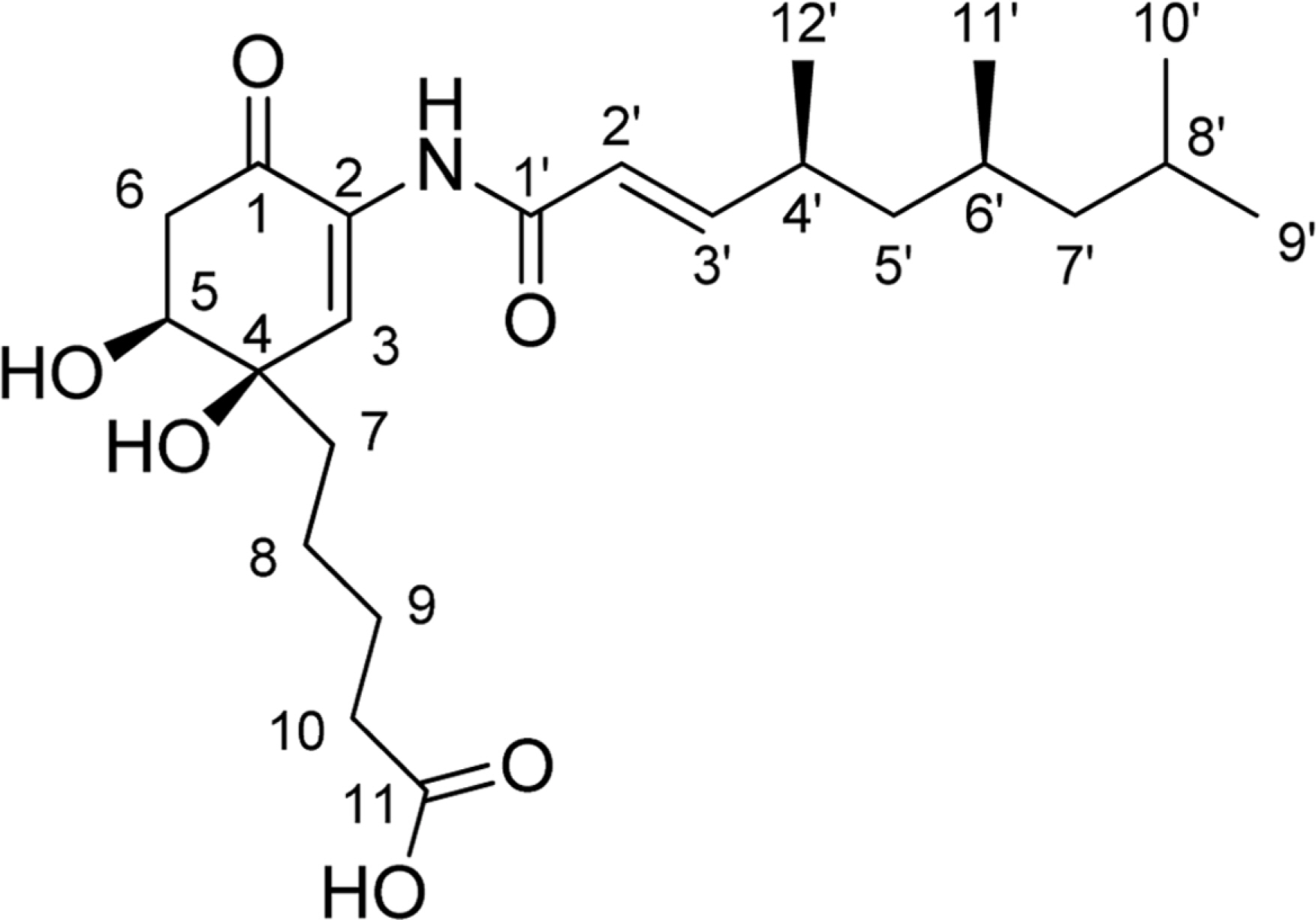
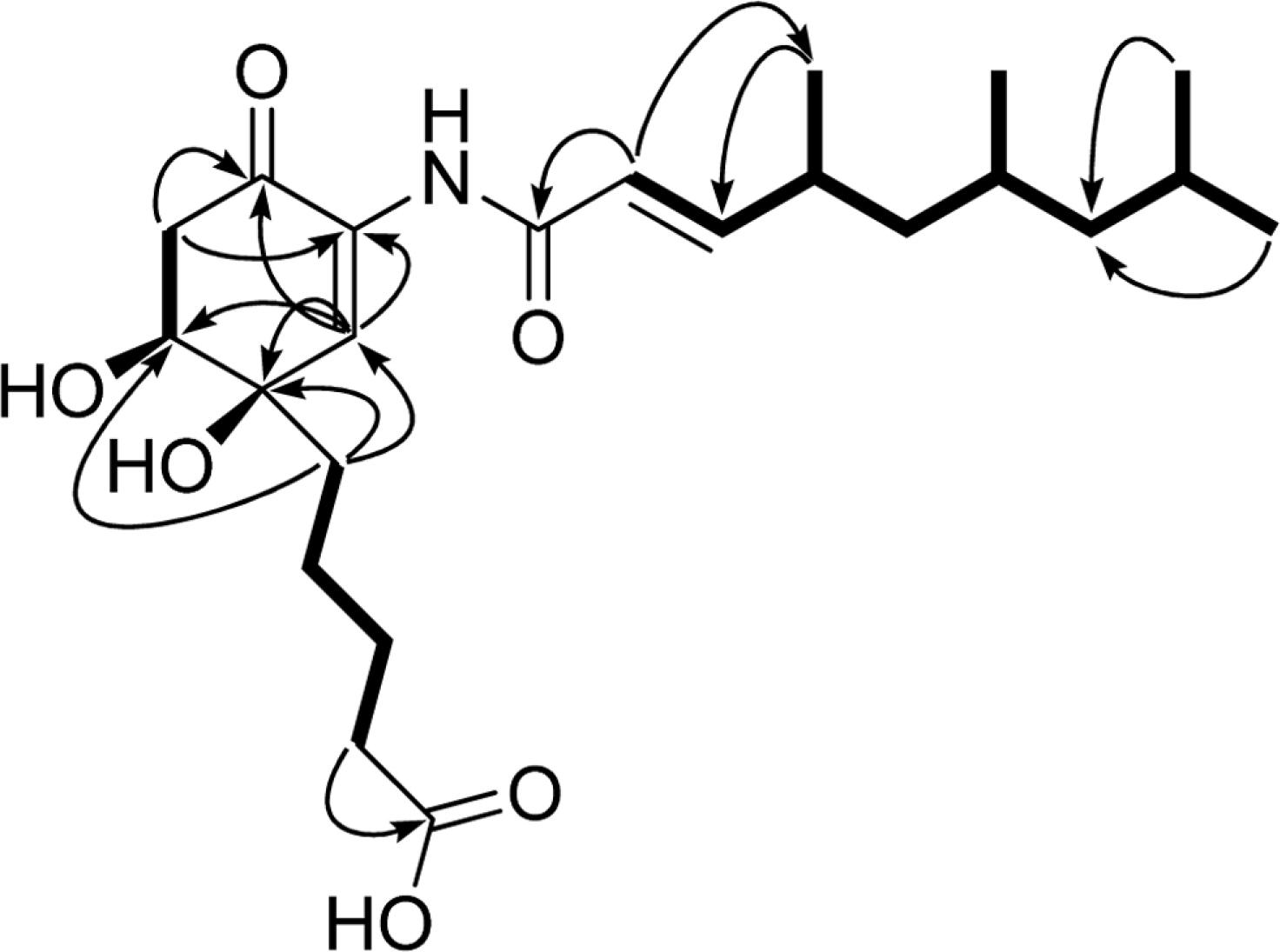
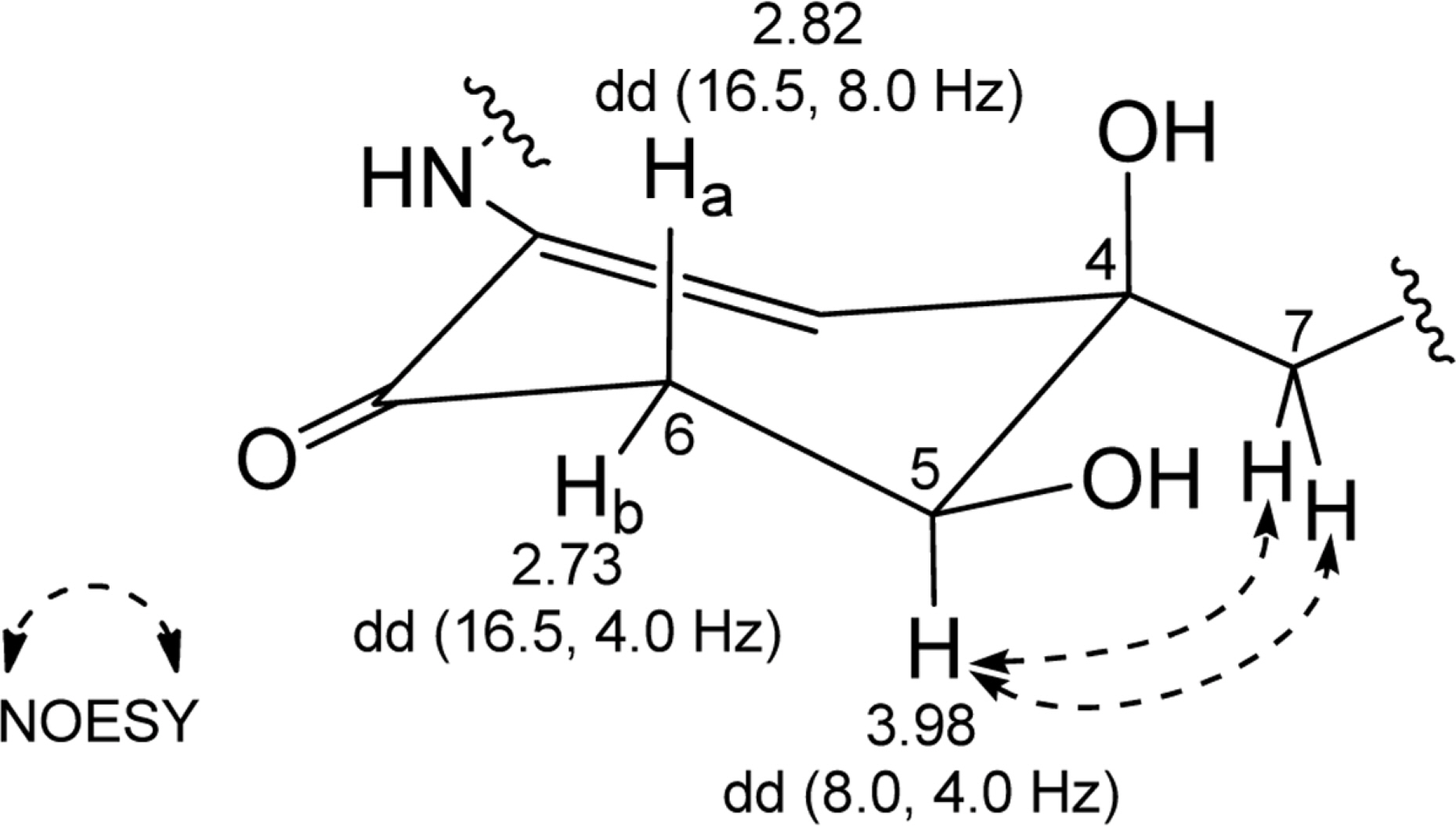
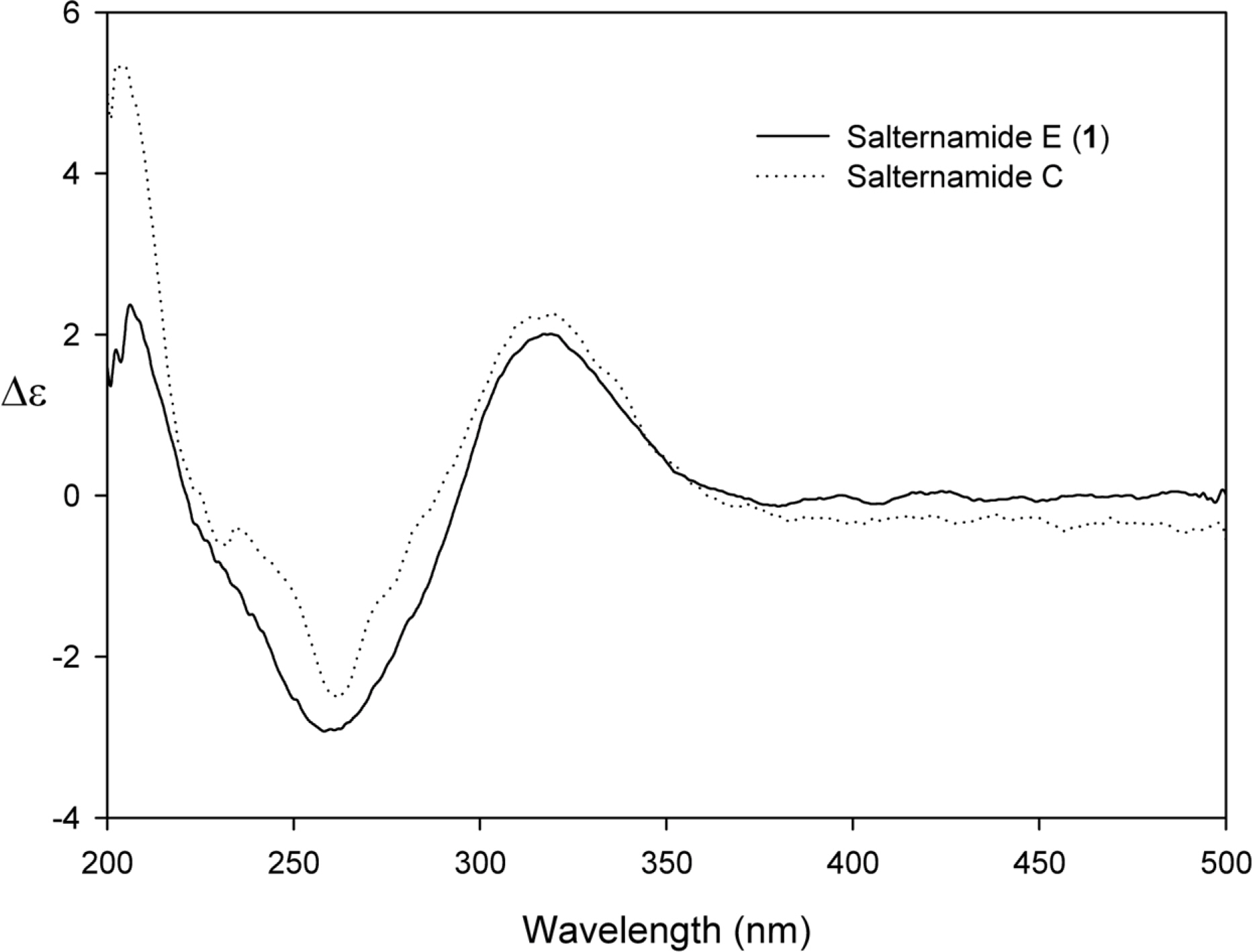
- Comprehensive chemical analysis of extracts and fractions of marine actinomycete strains led to the discovery of a new minor secondary metabolite, salternamide E (1), from a saltern-derived halophilic Streptomyces strain. The planar structure of salternamide E (1) was elucidated by a combinational analysis of spectroscopic data including NMR, MS, UV, and IR. The absolute configuration of salternamide E (1) was determined by circular dichroism spectroscopic analysis. Salternamide E displayed weak cytotoxicity against various human carcinoma cell lines.
Figure
Reference
-
(1). Fenical W., Jensen P. R.Nat. Chem. Biol. 2006; 2:666–673.(2). Moon K., Chung B., Shin Y., Rheingold A. L., Moore C. E., Park S. J., Park S., Lee S. K., Oh K.-B., Shin J., Oh D.-C. J.Nat. Prod. 2015; 78:524–529.(3). Hou Y., Braun D. R., Michel C. R., Klassen J. L., Adnani N., Wyche T. P., Bugni T. S.Anal. Chem. 2012; 84:4277–4283.(4). Forner D., Berrué F., Correa H., Duncan K., Kerr R. G.Anal. Chim. Acta. 2013; 805:70–79.(5). Yang J. Y., Sanchez L. M., Rath C. M., Liu X., Boudreau P. D., Bruns N., Glukhov E., Wodtke A., de Felicio R., Fenner A., Wong W. R., Linington R. G., Zhang L., Debonsi H. M., Gerwick W. H., Dorrestein P. C. J.Nat. Prod. 2013; 76:1686–1699.(6). Um S., Kim Y.-J., Kwon H., Wen H., Kim S.-H., Kwon H. C., Park S., Shin J., Oh D.-C. J.Nat. Prod. 2013; 76:873–879.(7). Um S., Choi T. J., Kim H., Kim B. Y., Kim S.-H., Lee S. K., Oh K.-B., Shin J., Oh D.-C. J.Org. Chem. 2013; 78:12321–12329.(8). Kwon Y., Kim S.-H., Shin Y., Bae M., Kim B.-Y., Lee S.K.; Oh, K.-B.; Shin, J.; Oh, D.-C. Mar. Drugs. 2014; 12:2326–2340.(9). Bae M., Kim H., Moon K., Nam S.-J., Shin J., Oh K.-B., Oh D.-C.Org. Lett. 2015; 17:712–715.(10). Kim S.-H., Shin Y., Lee S.-H., Oh K.-B., Lee S. K., Shin J., Oh D.-C. J.Nat. Prod. 2015; 78:836–843.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Violapyrone J, alpha-Pyrone Derivative from a Marine-derived Actinomycetes, Streptomyces sp.
- Deuteromethylactin B from a Freshwater-derived Streptomyces sp.
- Anti-Inflammatory Effect of Violapyrones B and C from a Marine-derived Streptomyces sp.
- Optimal Conditions for Antimicrobial Metabolites Production from a New Streptomyces sp. RUPA-08PR Isolated from Bangladeshi Soil
- Calyxaprenols A-D, New Merohexaprenoid Metabolites from the Marine Sponge Calyx sp.