Nat Prod Sci.
2015 Dec;21(4):255-260. 10.20307/nps.2015.21.4.255.
Graphiumins I and J, New Thiodiketopiperazines from the Marine-derived Fungus Graphium sp. OPMF00224
- Affiliations
-
- 1Graduate School of Pharmaceutical Sciences, Kitasato University, 5-9-1 Shirokane, Minato-ku, Tokyo 108-8641, Japan. tomodah@pharm.kitasato-u.ac.jp
- 2OP BIO FACTORY Co., Ltd., 5 Uruma Sandpit, Okinawa 904-2234, Japan.
- KMID: 2312906
- DOI: http://doi.org/10.20307/nps.2015.21.4.255
Abstract
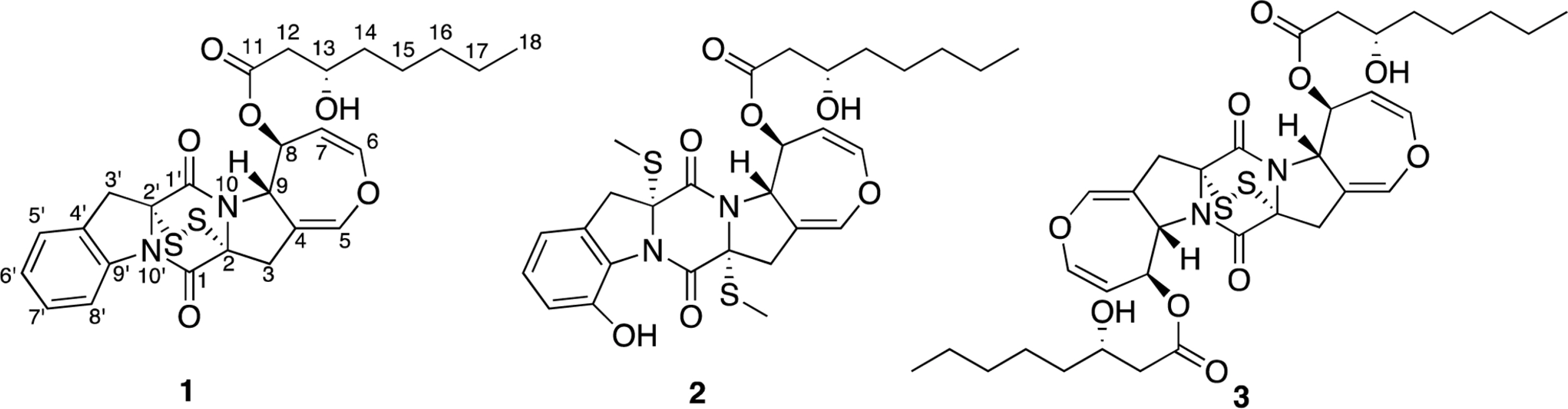
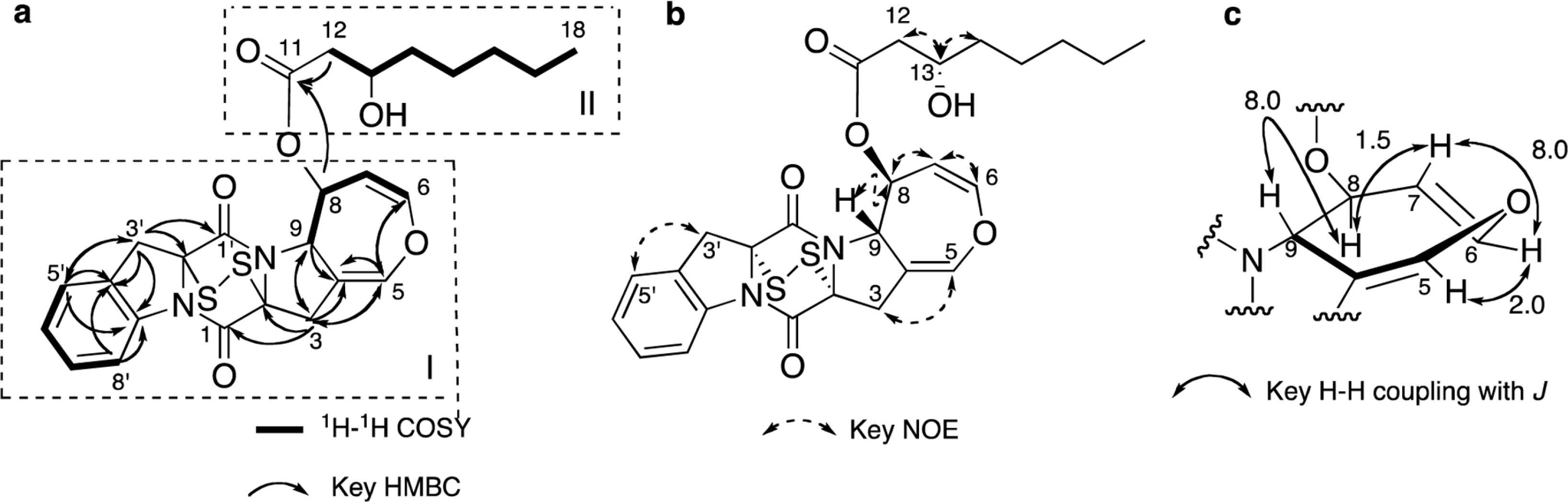
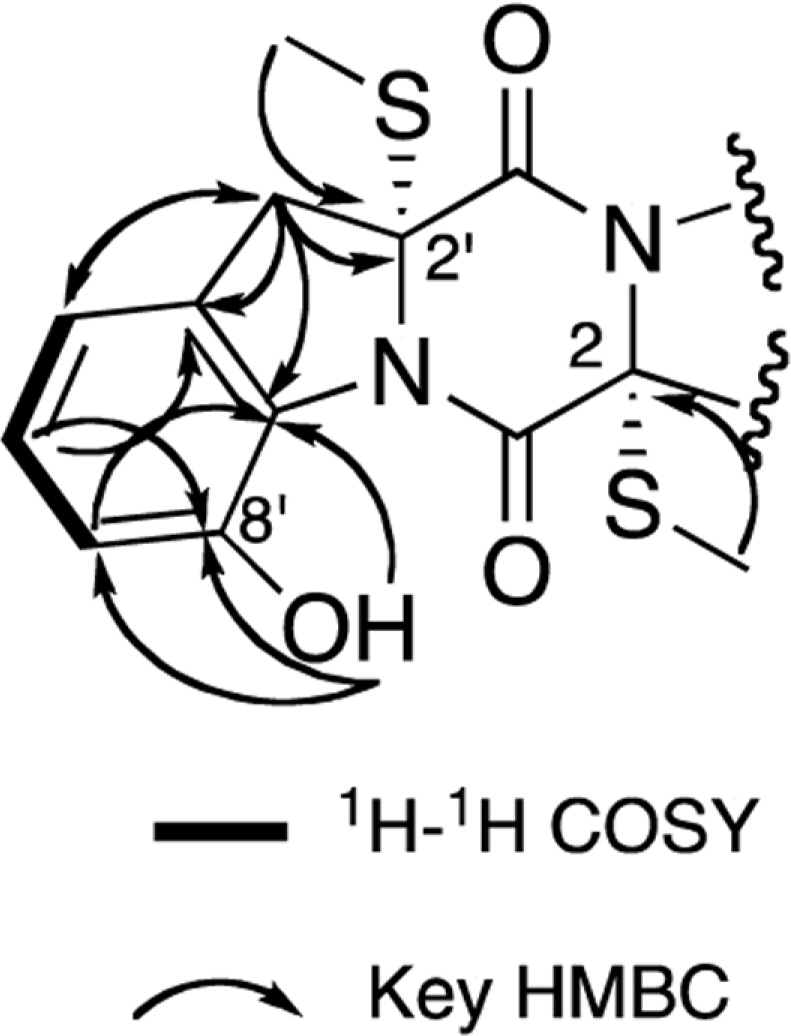
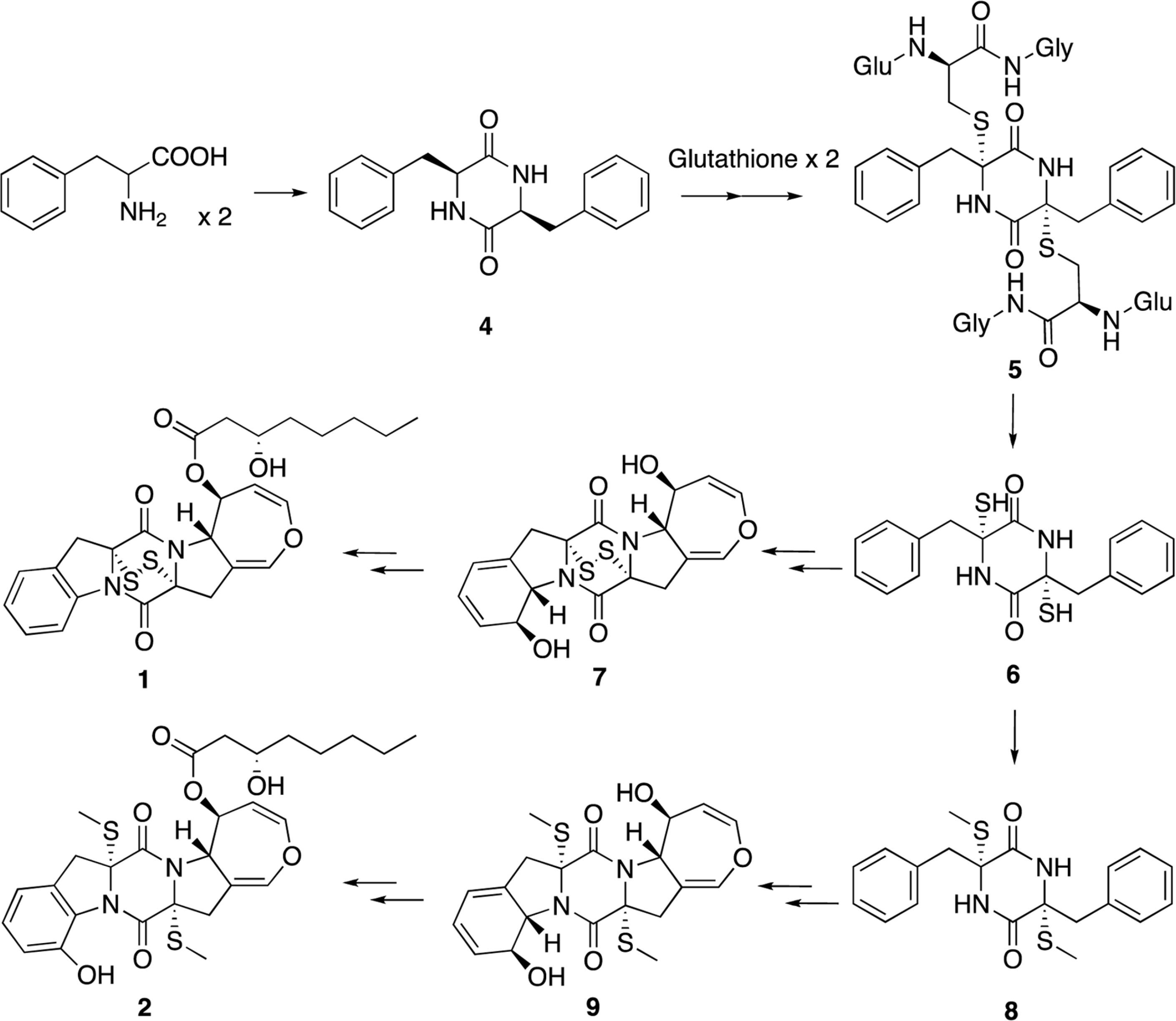
- Two new thiodiketopiperazines (TDKPs), designated graphiumins I (1) and J (2), were isolated from the culture broth of the marine-derived fungus Graphium sp. OPMF00224 by solvent extraction, silica gel column chromatography, and HPLC. Their absolute structures were elucidated by spectroscopic analyses (1D and 2D NMR data, ROESY correlations, and CD data) and chemical methods. They were found to be structurally rare TDKPs with a phenylalanine-derived indolin substructure. Compounds 1 and 2 inhibited yellow pigment production by methicillin-resistant Staphylococcus aureus (MRSA) with IC50 values of 63.5 and 76.5 microg/ml, respectively, without inhibiting its growth, even at 250 microg/ml.
MeSH Terms
Figure
Reference
-
(1). Centers for Disease Control and Prevention (CDC). MMWR Morb. Mortal. Wkly. Rep. 1997; 46:765–766.(2). Hiramatsu K., Hanaki H., Ino T., Yabuta K., Oguri T., Tenover F. C. J.Antimicrob. Chemother. 1997; 40:135–136.(3). Marshall J. H., Wilmoth G. J. J.Bacteriol. 1981; 147:900–913.(4). Marshall J. H., Wilmoth G. J. J.Bacteriol. 1981; 147:914–919.(5). Clauditz A., Resch A., Wieland K. P., Peschel A., Götz F.Infect. Immun. 2006; 74:4950–4953.(6). Liu G. Y., Essex A., Buchanan J. T., Datta V., Hoffman H. M., Bastian J. F., Fierer J., Nizet V. J.Exp. Med. 2005; 202:209–215.(7). Liu C. I., Liu G. Y., Song Y., Yin F., Hensler M. E., Jeng W. Y., Nizet V., Wang A. H., Oldfield E.Science. 2008; 319:1391–1394.(8). Song Y., Liu C. I., Lin F. Y., No J. H., Hensler M. E., Liu Y. L., Jeng W. Y., Low J., Liu G. Y., Nizet V., Wang A. H., Oldfield E. J.Med. Chem. 2009; 52:3869–3880.(9). Liu C. I., Jeng W. Y., Chang W. J., Ko T. P., Wang A. H. J.Biol. Chem. 2012; 287:18750–18757.(10). Lee J. H., Cho H. S., Kim Y., Kim J. A., Banskota S., Cho M. H., Lee J.Appl. Microbiol. Biotechnol. 2013; 97:4543–4552.(11). Lee J. H., Park J. H., Cho M. H., Lee J.Curr. Microbiol. 2012; 65:726–732.(12). Sakai K., Koyama N., Fukuda T., Mori Y., Onaka H., Tomoda H.Biol. Pharm. Bull. 2012; 35:48–53.(13). Fukuda T., Nagai K., Tomoda H. J.Nat. Prod. 2012; 75:2228–2231.(14). Fukuda T., Shimoyama K., Nagamitsu T., Tomoda H. J.Antibiot. 2014; 67:445–450.(15). Fukuda T., Shinkai M., Sasaki E., Nagai K., Kurihara Y., Kanamoto A., Tomoda H. J.Antibiot. 2015; DOI: doi: 10.1038/ja.2015.41.(16). Wang J. M., Jiang N., Ma J., Yu S. S., Tan R. X., Dai J. G., Si Y. K., Ding G. Z., Ma S. G., Qu J., Fang L., Du D.Tetrahedron. 2013; 69:1195–1201.(17). Nagai K., Doi T., Sekiguchi T., Namatame I., Sunazuka T., Tomoda H., Omura S., Takahashi T. J.Comb. Chem. 2006; 8:103–109.(18). Hegde V. R., Dai P., Patel M., Das P. R., Puar M. S.Tetrahedron Lett. 1997; 38:911–914.(19). Neuss N., Nagarajan R., Molloy B. B., Huckstep L. L.Tetrahedron Lett. 1968; 9:4467–4471.(20). Guo C. J., Yeh H. H., Chiang Y. M., Sanchez J. F., Chang S. L., Bruno K. S., Wang C. C. J.Am. Chem. Soc. 2013; 135:7205–7213.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pestalotiolide A, a New Antiviral Phthalide Derivative from a Soft Coral-derived Fungus Pestalotiopsis sp.
- New Production of Antibacterial Polycyclic Quinazoline Alkaloid, Thielaviazoline, from Anthranilic Acid by the Marine-Mudflat-Derived Fungus Thielavia sp.
- Penidioxolanes A and B, 1,3-Dioxolane Containing Azaphilone Derivatives from Marine-derived Penicillium sp. KCB12C078
- Calyxaprenols A-D, New Merohexaprenoid Metabolites from the Marine Sponge Calyx sp.
- Long-Term Investigation of Marine-Derived Aspergillus Diversity in the Republic of Korea