Nat Prod Sci.
2015 Dec;21(4):227-230. 10.20307/nps.2015.21.4.227.
Pestalotiolide A, a New Antiviral Phthalide Derivative from a Soft Coral-derived Fungus Pestalotiopsis sp.
- Affiliations
-
- 1Key Laboratory of Marine Drugs, The ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, People's Republic of China. shaochanglun@163.com
- KMID: 2312900
- DOI: http://doi.org/10.20307/nps.2015.21.4.227
Abstract
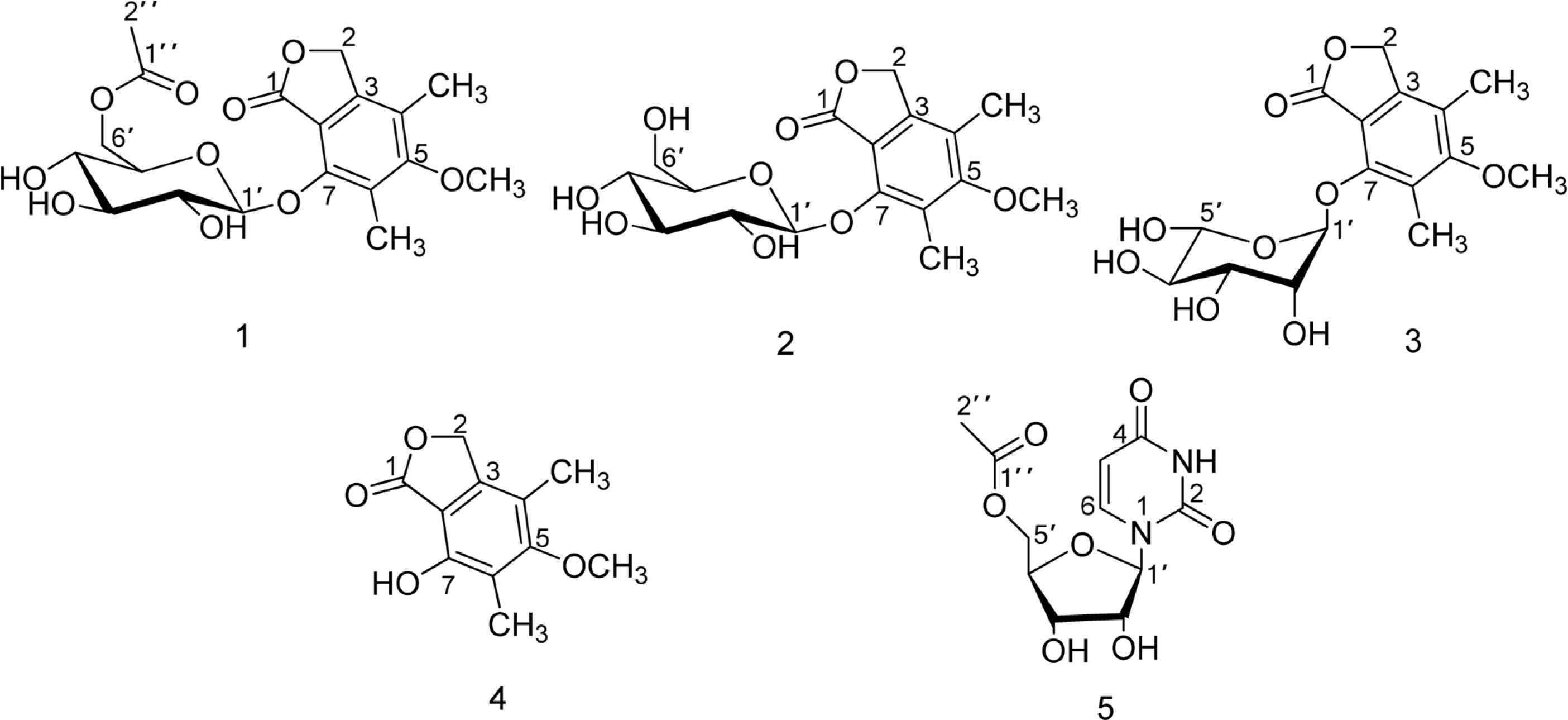
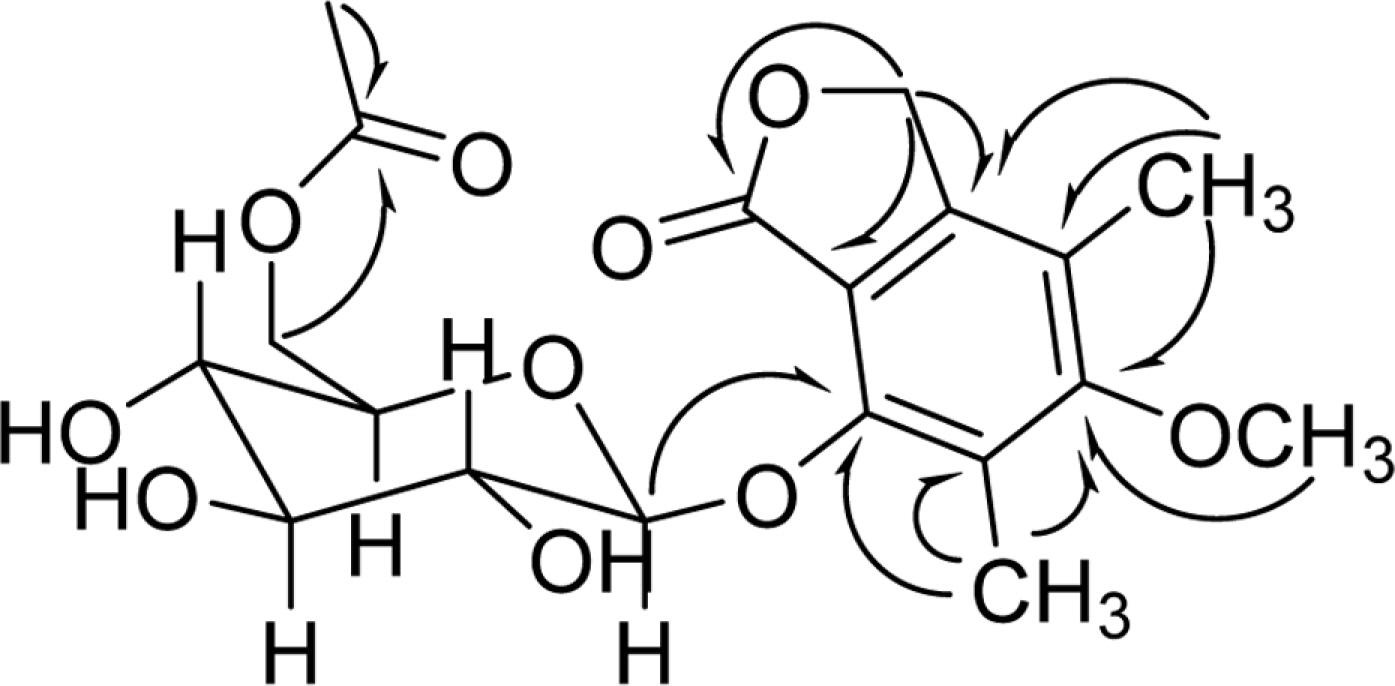
- Chemical investigation of the fermentation broth of a Soft Coral-Derived fungus Pestalotiopsis sp., led to the isolation of a new phthalide derivative, pestalotiolide A (1), three known analogues (2, 3 and 4), along with 5'-O-acetyl uridine (5) first isolated as a natural product. The structure of the new compound (1) was established by comprehensive spectroscopic analysis and chemical methods. Compounds 1 - 4 possessed varying degrees of antiviral activities, which was reported for the first time. Compared to the positive control ribavirin (IC50 = 418.0 microM), pestalotiolide A (1) exhibited significant anti-EV71 activity in vitro, with an IC50 value of 27.7 microM. Furthermore, the preliminary structure-activity relationship of antiviral activities was also discussed.
MeSH Terms
Figure
Reference
-
(1). Yang X. L., Zhang J. Z., Luo D. Q.Nat. Prod. Rep. 2012; 29:622–641.(2). Xu J., Ebada S. S., Proksch P.Fungal. Divers. 2010; 44:15–31.(3). Li J., Li L., Si Y., Jiang X., Guo L., Che Y.Org. Lett. 2011; 13:2670–2673.(4). Klaiklay S., Rukachaisirikul V., Tadpetch K., Sukpondma Y., Phongpaichit S., Buatong J., Sakayaroj J.Tetrahedron. 2012; 68:2299–2305.(5). Beekman A. M., Barrow R. A. J.Nat. Prod. 2013; 76:2054–2059.(6). Wu B., Wu X., Sun M., Li M.Mar. Drugs. 2013; 11:2713–2721.(7). Wang J., Wei X., Lu X., Xu F., Wan J., Lin X., Zhou X., Liao S., Yang B., Tu Z., Liu Y.Tetrahedron. 2014; 70:9695–9701.(8). Arunpanichlert J., Rukachaisirikul V., Phongpaichit S., Supaphon O., Sakayaroj J.Tetrahedron. 2015; 71:882–888.(9). Wang J., Wei X., Qin X., Chen H., Lin X., Zhang T., Yang X., Liao S., Yang B., Liu J., Zhou X., Tu Z., Liu Y.Phytochem. Lett. 2015; 12:59–62.(10). Wei M. Y., Li D., Shao C. L., Deng D. S., Wang C. Y.Mar. Drugs. 2013; 11:1050–1060.(11). Chen G., Tian L., Wu H. H., Bai J., Lu X., Xu Y., Pei Y. H. J.Asian Nat. Prod. Res. 2012; 14:759–763.(12). Simeó Y., Sinisterra J. V., Alcántara A. R.Green Chem. 2009; 11:855–862.(13). Schneider G., Anke H., Sterner O.Nat. Prod. Lett. 1997; 10:133–138.(14). Xing J. G., Deng H. Y., Luo D. Q. J.Asian Nat. Prod. Res. 2011; 13:1069–1073.(15). Grassauer A., Weinmuellner R., Meier C., Pretsch A., Prieschl-Grassauer E., Unger H.Virol. J. 2008; 5:107.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pestalotiopsis kaki sp. nov., a Novel Species Isolated from Persimmon Tree (Diospyros kaki) Bark in Korea
- Xylaroisopimaranin A, a New Isopimarane Derivative from an Endophytic Fungus Xylaralyce sp.
- First Case of Fungal Corneal Ulcer Caused by Pestalotiopsis mangiferae
- Delayed Contact Dermatitis to Coral
- Graphiumins I and J, New Thiodiketopiperazines from the Marine-derived Fungus Graphium sp. OPMF00224