Lab Med Online.
2014 Oct;4(4):198-202. 10.3343/lmo.2014.4.4.198.
Evaluation of the Verigene Warfarin Metabolism Nucleic Acid Test Kit for the Rapid Detection of CYP2C9 and VKORC1 Polymorphisms
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. chez@catholic.ac.kr
- 2Catholic Laboratory Development and Evaluation Center, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department of Internal Medicine, Division of Cardiology, Seoul St. Mary's Hospital, Seoul, Korea.
- KMID: 2312256
- DOI: http://doi.org/10.3343/lmo.2014.4.4.198
Abstract
- BACKGROUND
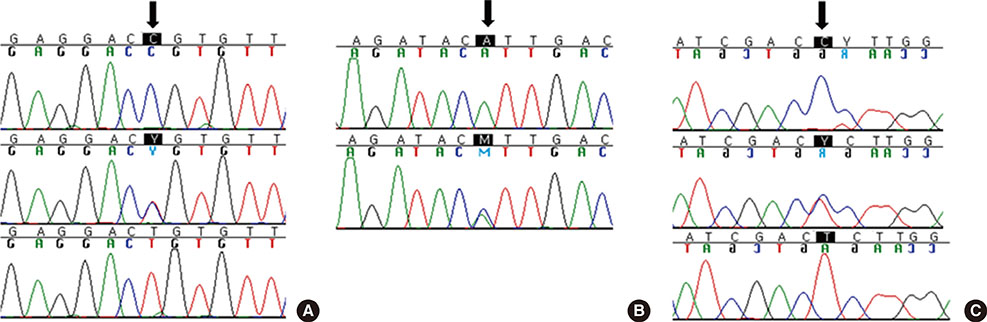
Warfarin is a widely used oral agent for anticoagulation therapy. Warfarin has a narrow therapeutic index and a wide variation in the interindividual therapeutic dosage. Recently, genotypes of CYP2C9 and VKORC1 have been found to account for 30-40% of the warfarin dosing variability, and a variety of commercial genotyping assays are being introduced. In this study, we evaluated the Verigene Warfarin Metabolism Nucleic Acid test (Verigene Warfarin assay; Nanosphere, USA) for its accuracy and clinical utility in genotyping CYP2C9*2, CYP2C9*3, and VKORC1 1173C>T.
METHODS
We compared the Verigene Warfarin assay with direct sequencing for accuracy in determining the genotypes of CYP2C9*2, CYP2C9*3, and VKORC1 1173C>T using 50 patient samples and 3 commercial DNA samples with known genotypes. The method was also evaluated for turn-around time, hands-on time, and feasibility.
RESULTS
The Verigene Warfarin assay demonstrated 100% accuracy for identifying CYP2C9*2, CYP2C9*3, and VKORC1 1173C>T. The turn-around time and hands-on time were 3 hr and 2 min, respectively. The no-call error rate at first attempt was estimated to be 2%.
CONCLUSIONS
The Verigene Warfarin assay provides rapid and accurate genotype results. Considering there are only a few steps requiring manual intervention, it would be feasible to implement this assay even in clinical laboratories that lack considerable expertise in molecular diagnostics.
Figure
Reference
-
1. Cha YJ, editor. Hematology. 2nd ed. Seoul: Korean Society of Hematology;2011. p. 654.2. Ma C, Zhang Y, Xu Q, Yang J, Zhang Y, Gao L, et al. Influence of warfarin dose-associated genotypes on the risk of hemorrhagic complications in Chinese patients on warfarin. Int J Hematol. 2012; 96:719–728.
Article3. Avery PJ, Jorgensen A, Hamberg AK, Wadelius M, Pirmohamed M, Kamali F. A proposal for an individualized pharmacogenetics-based warfarin initiation dose regimen for patients commencing anticoagulation therapy. Clin Pharmacol Ther. 2011; 90:701–706.
Article4. Aquilante CL, Langaee TY, Lopez LM, Yarandi HN, Tromberg JS, Mohuczy D, et al. Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin Pharmacol Ther. 2006; 79:291–302.
Article5. D'Andrea G, D'Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005; 105:645–649.6. Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005; 106:2329–2333.
Article7. Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics. 2002; 12:251–263.
Article8. Finkelman BS, Gage BF, Johnson JA, Brensinger CM, Kimmel SE. Genetic warfarin dosing: tables versus algorithms. J Am Coll Cardiol. 2011; 57:612–618.9. Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005; 352:2285–2293.
Article10. Cho HJ, Sohn KH, Park HM, Lee KH, Choi B, Kim S. Factors affecting the interindividual variability of warfarin dose requirement in adult Korean patients. Pharmacogenomics. 2007; 8:329–337.
Article11. Maurice CB, Barua PK, Simses D, Smith P, Howe JG, Stack G. Comparison of assay systems for warfarin-related CYP2C9 and VKORC1 genotyping. Clin Chim Acta. 2010; 411:947–954.
Article12. Buchan BW, Peterson JF, Cogbill CH, Anderson DK, Ledford JS, White MN, et al. Evaluation of a microarray-based genotyping assay for the rapid detection of cytochrome P450 2C19 *2 and *3 polymorphisms from whole blood using nanoparticle probes. Am J Clin Pathol. 2011; 136:604–608.
Article13. King CR, Porche-Sorbet RM, Gage BF, Ridker PM, Renaud Y, Phillips MS, et al. Performance of commercial platforms for rapid genotyping of polymorphisms affecting warfarin dose. Am J Clin Pathol. 2008; 129:876–883.
Article14. Langley MR, Booker JK, Evans JP, McLeod HL, Weck KE. Validation of clinical testing for warfarin sensitivity: comparison of CYP2C9-VKORC1 genotyping assays and warfarin-dosing algorithms. J Mol Diagn. 2009; 11:216–225.15. Babic N, Haverfield EV, Burrus JA, Lozada A, Das S, Yeo KT. Comparison of performance of three commercial platforms for warfarin sensitivity genotyping. Clin Chim Acta. 2009; 406:143–147.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Warfarin-Induced Bleeding in a Patient with CYP2C9 and VKORC1 Gene Polymorphism, Detected by a Point-of-Care Gene Test Device
- A child with Kawasaki disease and genetic warfarin sensitivity from CYP2C9 and VKORC1 gene variants
- VKORC1 and CYP2C9 Genotype Variations in Relation to Warfarin Dosing in Korean Stroke Patients
- A Case of Intolerance to Warfarin Dosing in an Intermediate Metabolizer of CYP2C9
- Extremely Elevated International Normalized Ratio of Warfarin in a Patient with CYP2C9*1/*3 and Thyrotoxicosis