Lab Med Online.
2013 Oct;3(4):198-212.
Analytical Performance of Wako and Sekisui Clinical Chemistry Assays on Hitachi LABOSPECT 008
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. phi@catholic.ac.kr
Abstract
- BACKGROUND
We evaluated the analytical performance of Wako assays for albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), complement C3 and C4, calcium, creatine kinase (CK), C-reactive protein (CRP), direct bilirubin (DBIL), iron, gamma-glutamyl transferase (GGT), HDL cholesterol (HDLC), inorganic phosphorus (IP), LDL cholesterol (LDLC), total bilirubin (TBIL), total protein (TP), and uric acid (UA), as well as the performance of Sekisui assays for albumin, BUN, calcium, CRP, HDLC, IP, LDLC, TP, and UA by using Hitachi LABOSPECT 008 (Hitachi High-Tech Co., Japan).
METHODS
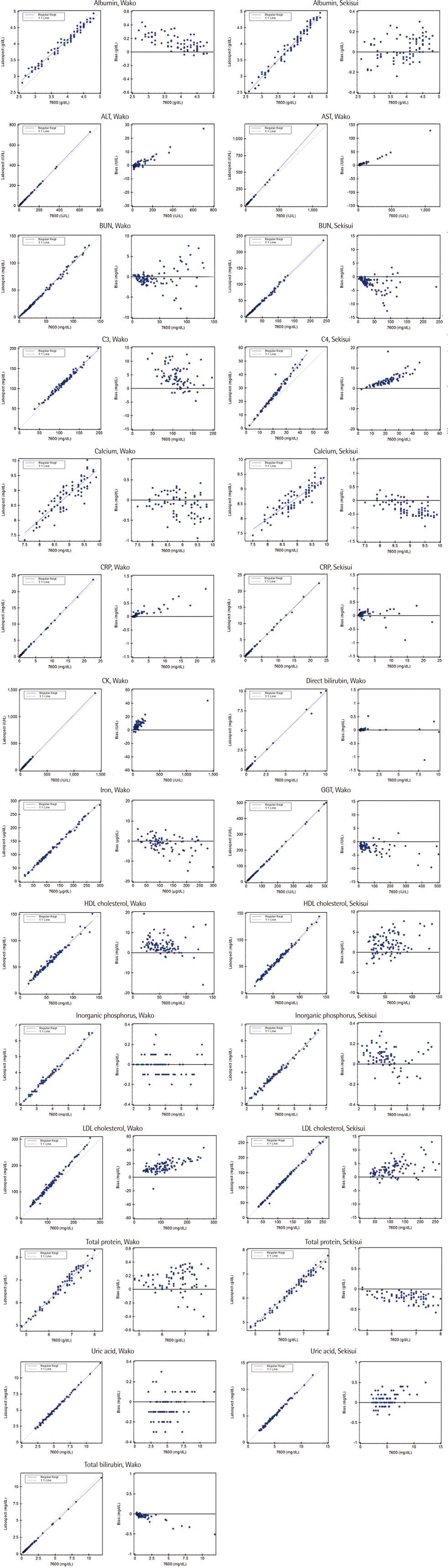
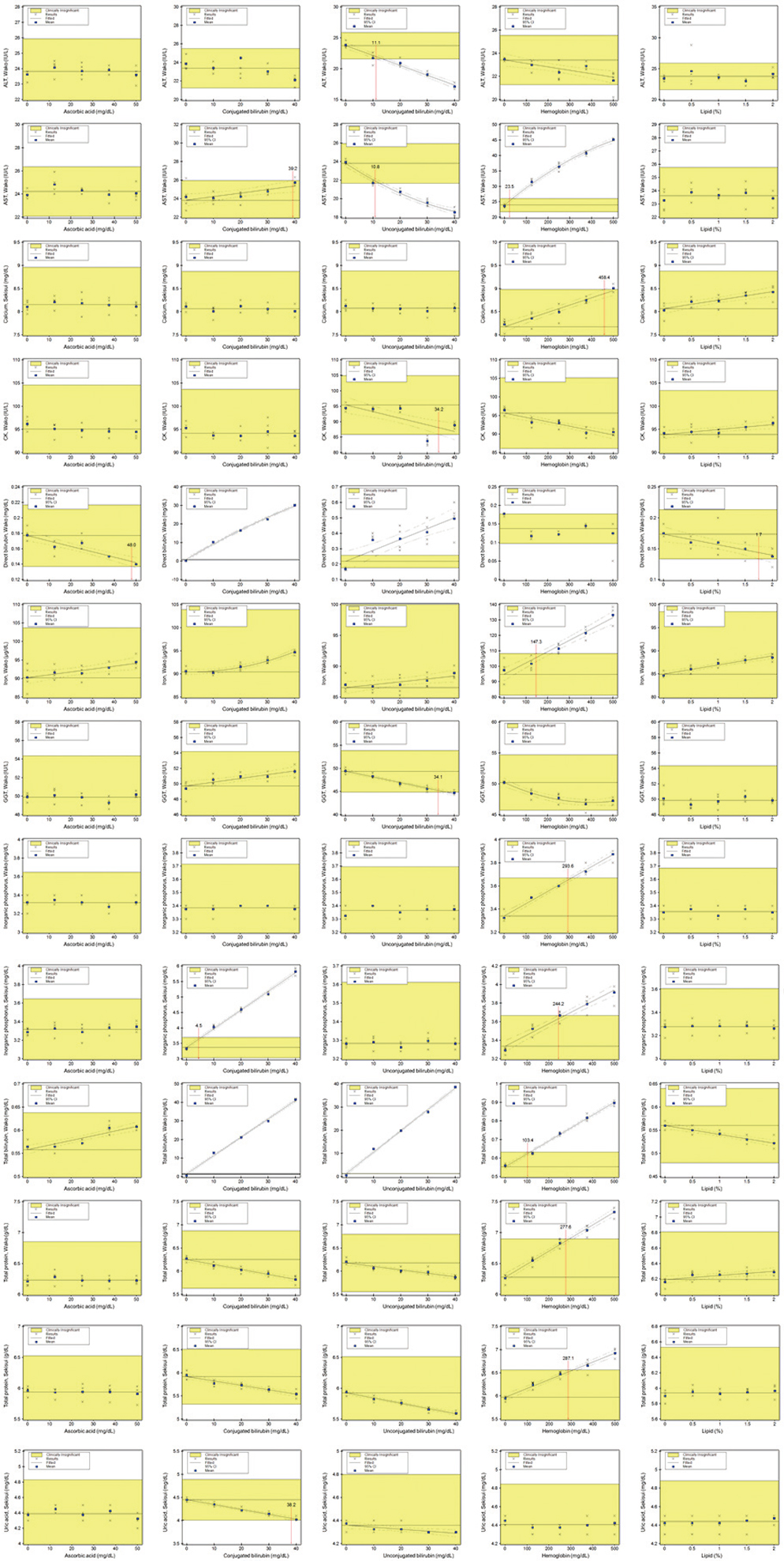
Liquid Assayed Multiqual Control (Bio-Rad Laboratories, USA) and pooled patients' sera were analyzed for 20 days. Wako linearity material (Wako Pure Chemical Industries, Ltd., Japan) and Sysmex Interference Check A Plus kit (Sysmex Co., Japan) were used to test linearity and interference, respectively. Concentrations of the target analytes were measured using Hitachi LABOSPECT 008 in 100 residual patient specimens and compared to those in Pureauto S series reagent (Sekisui Medical, Japan), which were measured using Hitachi 7600 (Hitachi High-Tech Co., Japan).
RESULTS
Total coefficients of variation (CVs) for the tested analytes were 0.91-9.26% in Wako and 1.04-7.46% in Sekisui assays. Linearity was demonstrated up to the highest concentration within the analytical range in all the assays except for Wako albumin and Sekisui TP. Wako and Sekisui albumin, BUN, CRP, HDLC, and LDLC assays, and in Wako C3, C4, calcium, and UA assays showed no interference with the test concentrations used. All the tested assays, except for Wako AST, LDLC, and TP, and Sekisui calcium and TP, demonstrated comparability with comparative method for at least one medical decision level.
CONCLUSIONS
Our study results showed that the analytical performances of Wako and Sekisui chemistry assays evaluated using Hitachi LABOSPECT 008 had appropriate analytical performance for clinical use.
Keyword
MeSH Terms
-
Alanine Transaminase
Aspartate Aminotransferases
Bilirubin
Blood Urea Nitrogen
C-Reactive Protein
Calcium
Chemical Industry
Chemistry Techniques, Analytical
Chemistry, Clinical
Cholesterol, HDL
Cholesterol, LDL
Clinical Chemistry Tests
Complement C3
Creatine Kinase
Humans
Iron
Phosphorus
Transferases
Uric Acid
Alanine Transaminase
Aspartate Aminotransferases
Bilirubin
C-Reactive Protein
Calcium
Cholesterol, HDL
Cholesterol, LDL
Complement C3
Creatine Kinase
Iron
Phosphorus
Transferases
Uric Acid
Figure
Reference
-
1. Hitachi Clinical Chemistry Analytical Instrument LABOSPECT 008. Updated on Sep 2012. http://www.hitachi-hitec.com/science/medical/labospect_008.html.2. Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods. approved guideline-second edition. Wayne, PA: Clinical and Laboratory Standards Institute;2006.3. Westgard J, editor. Desirable biological variation database specifications. Updated on Jan 2012. http://www.westgard.com/biodatabase1.htm.4. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline, EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.5. Clinical and Laboratory Standards Institute. Method comparison and bias estimation using patient samples; approved guideline-Second edition, EP09-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2002.6. Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 5th ed. St. Louis: Elsevier Saunders;2011. p. 2131–2188.7. Jacques W, editor. Interpretation of diagnostic tests. 8th ed. Philadelphia: Lippincott Williams & Wilkins;2007. p. 8–30.8. Westgard J. Medical decision levels. Updated on Jan 2011. http://www.westgard.com/decision.htm.9. Clinical and Laboratory Standards Institute. Interference testing in clinical chemistry, EP07-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2005.10. Kang SY, Suh JT, Kim JH, Lee WI, Lee HJ. Comparison of high sensitivity C-reactive protein assay with a wide assay range. Korean J Lab Med. 2005; 25:227–233.11. Sisman AR, Küme T, Tas G, Akan P, Tuncel P. Comparison and evaluation of two C-reactive protein assays based on particle-enhanced immunoturbidimetry. J Clin Lab Anal. 2007; 21:71–76.
Article12. Zivkovic AM, Wiest MM, Nguyen UT, Davis R, Watkins SM, German JB. Effects of sample handling and storage on quantitative lipid analysis in human serum. Metabolomics. 2009; 5:507–516.
Article13. Clinical and Laboratory Standards Institute. Procedures for the handling and processing of blood specimens for common laboratory tests; approved guideline - Fourth edition, H18-A4. Wayne, PA: Clinical and Laboratory Standards Institute;2010.14. Ji JZ, Meng QH. Evaluation of the interference of hemoglobin, bilirubin, and lipids on Roche Cobas 6000 assays. Clin Chim Acta. 2011; 412:1550–1553.
Article15. Steen G, Vermeer HJ, Naus AJ, Goevaerts B, Agricola PT, Schoenmakers CH. Multicenter evaluation of the interference of hemoglobin, bilirubin and lipids on Synchron LX-20 assays. Clin Chem Lab Med. 2006; 44:413–419.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the Roche-Hitachi cobas 8000 c702 Chemistry Autoanalyzer
- Performance Evaluation of the JEOL BioMajesty JCA-BM6010/C Automated Clinical Chemistry Analyzer
- A Comparative Study of Biological and Analytical Variability of Automated Clinical Chemistry Tests
- Performance Evaluation of Automated Chemistry Analyser for Urine Chemistry Test
- Comparison of Two New Plastic Tubes (Sekisui INSEPACK and Green Cross Green Vac-Tube) with BD Vacutainer Tubes for 49 Analytes