Lab Med Online.
2011 Jan;1(1):43-50.
Analysis of Positive Flow Cytometric Crossmatch in Organ Transplantation
- Affiliations
-
- 1Department of Clinical Pathology, Kyungpook National University School of Medicine, Daegu, Korea. wormie@lycos.co.kr
Abstract
- BACKGROUND
Pretransplant HLA crossmatch is one of the most important parts in solid organ transplantation. Flow cytometic crossmatch (FCXM) is more sensitive than anti-human globulin enhanced complement dependent lymphocytotoxicity (AHG-CDC) in detecting anti-HLA antibodies. We compared the results of the two methods and analyzed the FCXM-positive cases in various aspects.
METHODS
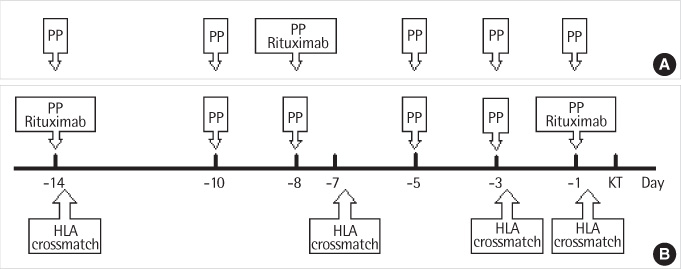
Sera from 212 patients were tested for the detection of anti-HLA antibodies by FCXM and 188 of them were also tested by AHG-CDC assay. The results were analyzed in relation to their histories of pregnancy, transfusion or organ transplantation and also according to the donor patient relationships. We compared the FCXM results obtained before and after desensitization therapy (using plasmapheresis and anti-CD20 antibody) in 5 sensitized patients.
RESULTS
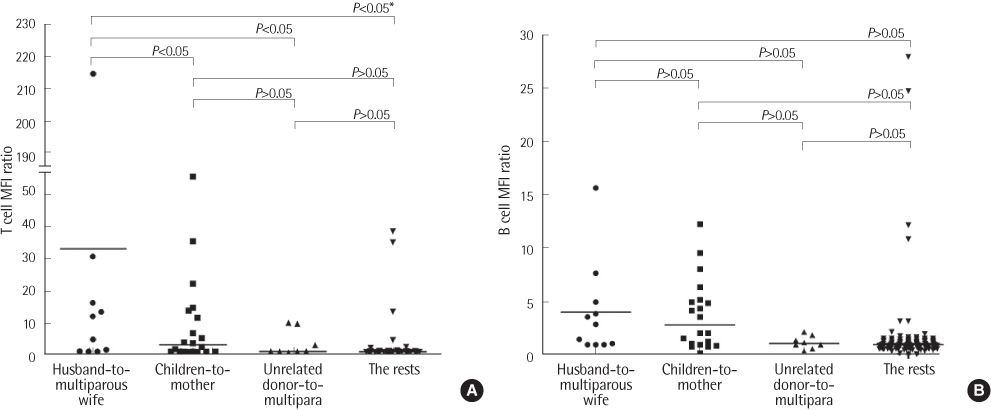
Concordance of the results between the two methods was 88.8% (167/188). FCXM results correlated with history of pregnancy, but not with that of transfusion. When the patients were divided into 4 groups according to donor-patient relationships, the T cell FCXM mean fluorescence intensity (MFI) ratio (sample/control) was significantly higher in the husband-to-multiparous wife group compared to the other 3 groups (children-to-mother, unrelated donor-to-multipara, and the rests). After desensitization therapy, MFI ratios of T cell FCXM decreased and those of B cell FCXM increased, probably due to rituximab effect, in all 5 patients.
CONCLUSIONS
FCXM using a MFI ratio, has a higher sensitivity than AHG-CDC in detection of donor specific antibodies. Also it can be useful in monitoring antibody levels during desensitization therapy.
MeSH Terms
Figure
Reference
-
1. Tinckam KJ, Chandraker A. Mechanisms and role of HLA and non-HLA alloantibodies. Clin J Am Soc Nephrol. 2006. 1:404–414.
Article2. Han KS, Park MH, editors. Transfusion medicine. 2006. 3rd ed. Seoul: Korea Medical Book Publisher;245–248.3. Roback JD, Combs MR, editors. Technical manual. 2008. 16th ed. Bethesda, Maryland: American Association of Blood Banks;562–566.4. Garovoy MR, Rheinschmidt MA, Bigos M, Perkins H, Colombe B, Feduska N, et al. Flow cytometry analysis: a high technology crossmatch technique facilitating transplantation. Transplant Proc. 1983. 15:1939–1944.5. Hoy T, Garner S, et al. Ormerod MG, editor. Further clinical applications. Flow cytometry. 2000. 3rd ed. New York: Oxford University Press;99–124.6. Robert AB. Darzynkiewicz Z, Robinson JP, editors. Flow cytometry crossmatching for solid organ transplantation. Method in cell biology: volume 41 flow cytometry part A. 1994. 2nd ed. San Diego: Academic Press;437–447.7. Oh EJ, Lee JH, Yang CW, Moon IS, Park YJ, Han KJ. Comparison of anti-HLA detecting methods: cytotoxicity, flow cytometric crossmatch, multiple antigen-ELISA, and single antigen-ELISA. J Korean Soc Transplant. 2008. 22:85–91.8. Won DI. Experimental application of whole blood flow cytometry to HLA crossmatch for renal transplantation. Korean J Lab Med. 2006. 26:45–51.
Article9. Ta M, Scornik JC. Improved flow cytometric detection of donor-specific HLA class II antibodies by heat inactivation. Transplantation. 2002. 73:1611–1614.
Article10. Zachary AA, Leffell MS. Detecting and monitoring human leukocyte antigen-specific antibodies. Hum Immunol. 2008. 69:591–604.
Article11. Robert AB, Howard MG. Keren FD, McCoy JP, editors. Clinical utility of flow cytometry in allogeneic transplantation. Flow cytometry in clinical diagnosis. 2001. 3rd ed. Chicago: ASCP Press;507–541.12. Christiaans MH, Overhof-de Roos F, Nieman F, van Hooff JP, van den Berg-Loonen EM. Donor-specific antibodies after transplantation by flow cytometry: relative change in fluorescence ratio most sensitive risk factor for graft survival. Transplantation. 1998. 65:427–433.
Article13. Le Bas-Bernardet S, Hourmant M, Valentin N, Paitier C, Giral-Classe M, Curry S, et al. Identification of the antibodies involved in B-cell crossmatch positivity in renal transplantation. Transplantation. 2003. 75:477–482.
Article14. Kotb M, Russell WC, Hathaway DK, Gaber LW, Gaber AO. The use of positive B cell flow cytometry crossmatch in predicting rejection among renal transplant recipients. Clin Transplant. 1999. 13:83–89.
Article15. Al-Muzairai IA, Mansour M, Almajed L, Alkanderi N, Alshatti N, Samhan M. Heat inactivation can differentiate between IgG and IgM antibodies in the pretransplant cross match. Transplant Proc. 2008. 40:2198–2199.
Article16. Vaidya S, Cooper TY, Avandsalehi J, Barnes T, Brooks K, Hymel P, et al. Improved flow cytometric detection of HLA alloantibodies using pronase: potential implications in renal transplantation. Transplantation. 2001. 71:422–428.
Article17. Lobo PI, Isaacs RB, Spencer CE, Pruett TL, Sanfey HA, Sawyer RG, et al. Improved specificity and sensitivity when using pronase-digested lymphocytes to perform flow-cytometric crossmatch prior to renal transplantation. Transpl Int. 2002. 15:563–569.
Article18. Bearden CM, Agarwal A, Book BK, Sidner RA, Gebel HM, Bray RA, et al. Pronase treatment facilitates alloantibody flow cytometric and cytotoxic crossmatching in the presence of rituximab. Hum Immunol. 2004. 65:803–809.
Article19. Book BK, Agarwal A, Milgrom AB, Bearden CM, Sidner RA, Higgins NG, et al. New crossmatch technique eliminates interference by humanized and chimeric monoclonal antibodies. Transplant Proc. 2005. 37:640–642.
Article20. Triulzi DJ, Kleinman S, Kakaiya RM, Busch MP, Norris PJ, Steele WR, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009. 49:1825–1835.
Article21. Bohmig GA, Regele H, Saemann MD, Exner M, Druml W, Kovarik J, et al. Role of humoral immune reactions as target for antirejection therapy in recipients of a spousal-donor kidney graft. Am J Kidney Dis. 2000. 35:667–673.
Article22. Mahanty HD, Cherikh WS, Chang GJ, Baxter-Lowe LA, Robert JP. Influence of pretransplant pregnancy on survival of renal allografts from living donors. Transplantation. 2001. 72:228–232.
Article23. Rosenberg JC, Jones B, Oh H. Accelerated rejection following offspring-to-mother and husband-to-wife transplants. Clin Transplant. 2004. 18:729–733.
Article24. Pollack MS, Trimarchi HM, Riley DJ, Casperson PR, Manyari LE, Suki WN. Shared cadaver donor-husband HLA class I mismatches as a risk factor for renal graft rejection in previously pregnant women. Hum Immunol. 1999. 60:1150–1155.
Article25. Vaidya S, Partlow D, Susskind B, Noor M, Barnes T, Gugliuzza K. Prediction of crossmatch outcome of highly sensitized patients by single and/or multiple antigen bead luminex assay. Transplantation. 2006. 82:1524–1528.
Article26. Magee CC, Mah H, Tinckam K, Wood I, Ji F, Powelson J. Successful living donor kidney transplantation across HLA and ABO incompatibilities. Nephrol Dial Transplant. 2007. 22:602–604.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Flow Cytometric AHG-CDC for HLA Crossmatch: A Pilot Study
- A Living Donor Liver Transplantation after Therapeutic Plasmapheresis in a Patient with Positive HLA Crossmatch
- Plasmapheresis in a Renal Transplant Patient with Positive Crossmatch only Detected by Flow Cytometry
- Causes of Positive Pretransplant Crossmatches in the Absence of Donor-Specific Anti-Human Leukocyte Antigen Antibodies: A Single-Center Experience
- False positive T-cell Cytotoxicity crossmatch results suggestive of autoantibodies in Korean Network for Organ Sharing crossmatch tests for deceased donor organ transplantation