Lab Anim Res.
2014 Sep;30(3):123-130. 10.5625/lar.2014.30.3.123.
Adverse effects of 4-tert-octylphenol on the production of oxytocin and hCG in pregnant rats
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources & Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. anbs@pusan.ac.kr
- 2Department of Obstetrics and Gynecology, Biomedical Research Institute, Pusan National University School of Medicine, Busan, Korea.
- KMID: 2312123
- DOI: http://doi.org/10.5625/lar.2014.30.3.123
Abstract
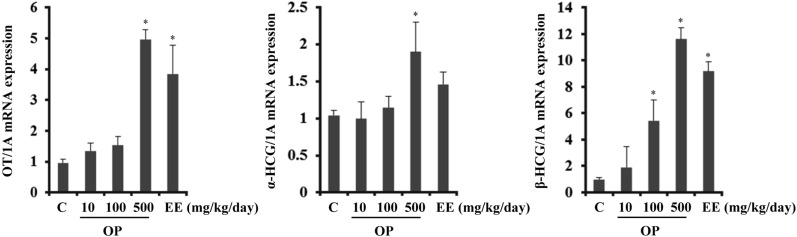
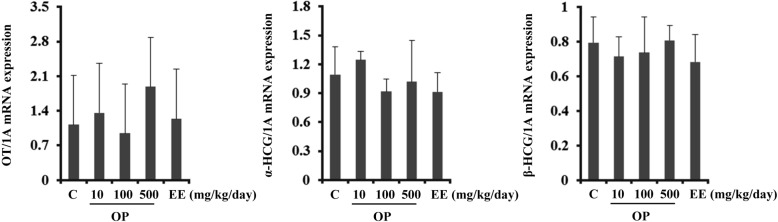
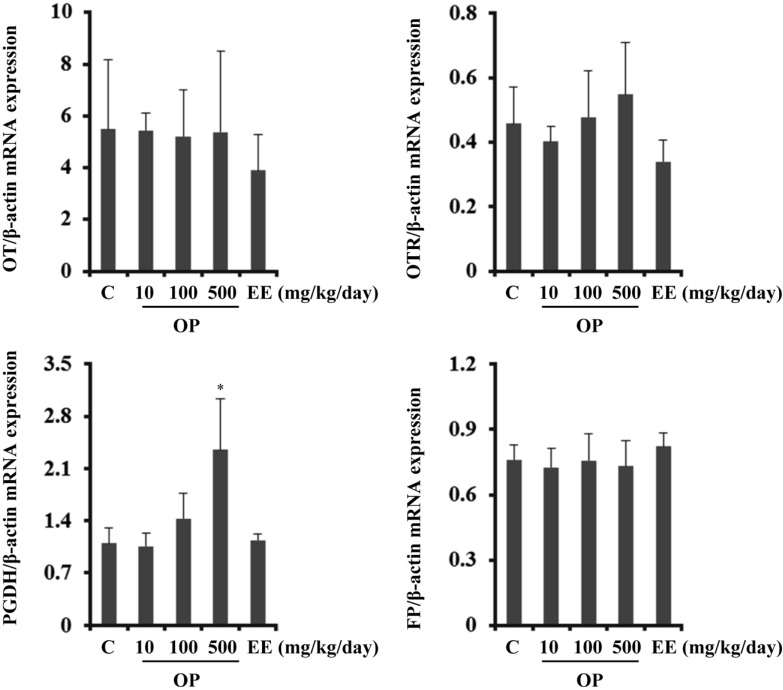
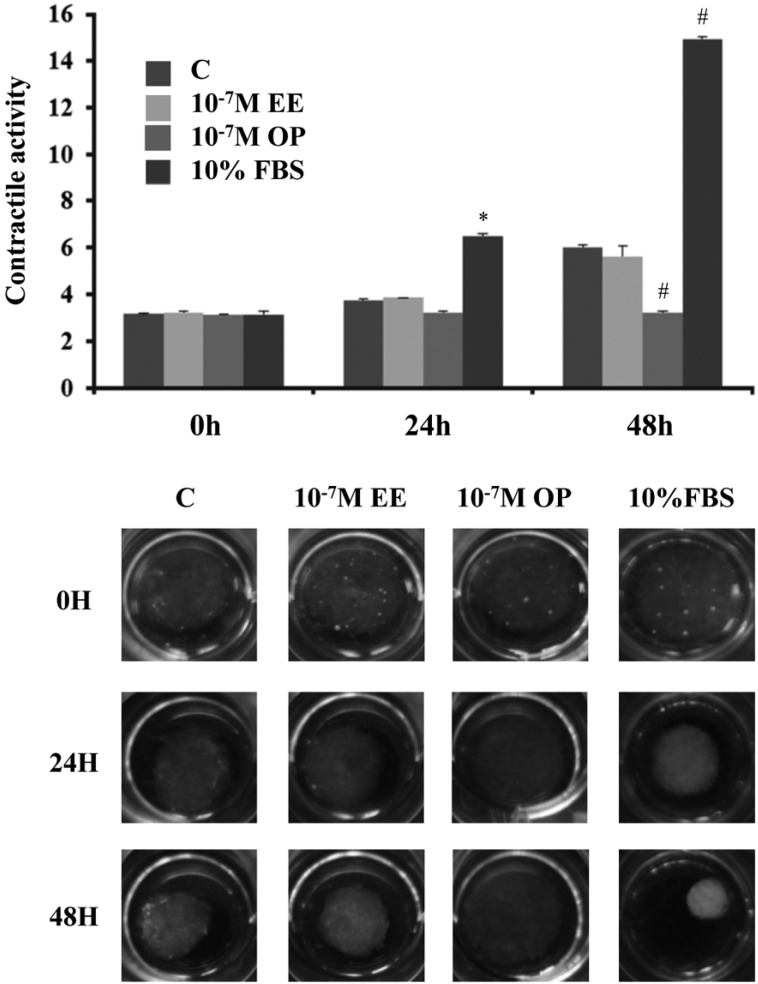
- Endocrine-disrupting chemicals (EDCs) are exogenous substances that alter the structure or function of the endocrine system. 4-Tert-octylphenol (OP) is one of the most representative EDCs and has estrogenic effects. In this study, we examined the effects of ethinyl estradiol (EE) and OP on the pituitary gland, placenta, and uterus of pregnant rats. Expression levels of human chorionic gonadotropin (hCG), oxytocin (OT), and contraction-associated proteins (CAPs) were determined, and uterine contractile activity was measured by uterine contraction assay. EE and OP both increased mRNA expression of OT and hCG in the pituitary gland but not the placenta. Since OT and hCG control uterine contraction, we next examined CAP expression in the uterus. Expression of 15-hydroxyprostaglandin-dehydrogenase (PGDH) was upregulated by OP, whereas expression of other CAPs was unaffected. To clarify the effect of OP on uterine contraction in pregnant rats, uterine contraction assay was performed. The 17beta-Estradiol (E2) did not affect contraction of primary uterine cells harvested from pregnant rats in a 3D collagen gel model. However, OP showed different effects from E2 by significantly reducing contraction activity. In summary, we demonstrated that OP interferes with regulation of OT and hCG in the pituitary gland as well as PGDH in the uterus, thereby reducing uterine contraction activity. This result differs from the action of endogenous E2. Collectively, these findings suggest that exposure to EDCs such as OP during pregnancycan reduce uterine contractile ability, which may result in contraction-associated adverse effects such as metratonia, bradytocia, and uterine leiomyomata.
Keyword
MeSH Terms
Figure
Reference
-
1. Nilsson R. Endocrine modulators in the food chain and environment. Toxicol Pathol. 2000; 28(3):420–431. PMID: 10862560.
Article2. Rudel RA, Perovich LJ. Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ (1994). 2009; 43(1):170–181. PMID: 20047015.
Article3. White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994; 135(1):175–182. PMID: 8013351.
Article4. An BS, Ahn HJ, Kang HS, Jung EM, Yang H, Hong EJ, Jeung EB. Effects of estrogen and estrogenic compounds, 4-tert-octylphenol, and bisphenol A on the uterine contraction and contraction-associated proteins in rats. Mol Cell Endocrinol. 2013; 375(1-2):27–34. PMID: 23664861.
Article5. Kota SK, Gayatri K, Jammula S, Kota SK, Krishna SV, Meher LK, Modi KD. Endocrinology of parturition. Indian J Endocrinol Metab. 2013; 17(1):50–59. PMID: 23776853.
Article6. Terranova PF. The female reproductive system. Medical Physiology. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins;2003. p. 667–683.7. White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994; 135(1):175–182. PMID: 8013351.
Article8. Hoermann R, Spoettl G, Moncayo R, Mann K. Evidence for the presence of human chorionic gonadotropin (hCG) and free beta-subunit of hCG in the human pituitary. J Clin Endocrinol Metab. 1990; 71(1):179–186. PMID: 1695224.9. Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Münstedt K, Rao CV, Lang U, Preissner KT. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002; 87(11):5290–5296. PMID: 12414904.
Article10. Shi QJ, Lei ZM, Rao CV, Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993; 132(3):1387–1395. PMID: 7679981.
Article11. Cronier L, Bastide B, Hervé JC, Délèze J, Malassiné A. Gap junctional communication during human trophoblast differentiation: influence of human chorionic gonadotropin. Endocrinology. 1994; 135(1):402–408. PMID: 8013377.
Article12. Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981; 50:465–495. PMID: 6267989.
Article13. Jenkins JS, Nussey SS. The role of oxytocin: present concepts. Clin Endocrinol (Oxf). 1991; 34(6):515–525. PMID: 1889133.
Article14. Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984; 150(6):734–741. PMID: 6093538.
Article15. Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001; 81(2):629–683. PMID: 11274341.16. Jagani N, Schulman H, Fleischer A, Mitchell J, Blattner P. Role of prostaglandin-induced cervical changes in labor induction. Obstet Gynecol. 1984; 63(2):225–229. PMID: 6582419.17. Nelson RJ. An introduction to behavioral endocrinology.Sinauer Associates. 2005. p. 100.18. Fuchs AR, Rollyson MK, Meyer M, Fields MJ, Minix JM, Randel RD. Oxytocin induces prostaglandin F2 alpha release in pregnant cows: influence of gestational age and oxytocin receptor concentrations. Biol Reprod. 1996; 54(3):647–653. PMID: 8835387.19. Dallot E, Pouchelet M, Gouhier N, Cabrol D, Ferré F, Breuiller-Fouché M. Contraction of cultured human uterine smooth muscle cells after stimulation with endothelin-1. Biol Reprod. 2003; 68(3):937–942. PMID: 12604645.
Article20. Dave JR, Culp SG, Liu L, Tabakoff B, Hoffman PL. Regulation of vasopressin and oxytocin synthesis in anterior pituitary and peripheral tissues. Adv Alcohol Subst Abuse. 1988; 7(3-4):231–234. PMID: 3223431.21. Lefebvre DL, Giaid A, Bennett H, Larivière R, Zingg HH. Oxytocin gene expression in rat uterus. Science. 1992; 256(5063):1553–1555. PMID: 1598587.
Article22. Engstrom T, Bratholm P, Vilhardt H, Christensen NJ. Effect of oxytocin receptor and beta2-adrenoceptor blockade on myometrial oxytocin receptors in parturient rats. Biol Reprod. 1999; 60(2):322–329. PMID: 9915997.23. Challis JR, Sloboda DM, Alfaidy N, Lye SJ, Gibb W, Patel FA, Whittle WL, Newnham JP. Prostaglandins and mechanisms of preterm birth. Reproduction. 2002; 124(1):1–17.24. Riemer RK, Heymann MA. Regulation of uterine smooth muscle function during gestation. Pediatr Res. 1998; 44(5):615–627. PMID: 9803440.
Article25. Kovalevskaya G, Genbacev O, Fisher SJ, Caceres E, O'Connor JF. Trophoblast origin of hCG isoforms: cytotrophoblasts are the primary source of choriocarcinoma-like hCG. Mol Cell Endocrinol. 2002; 194(1-2):147–155. PMID: 12242037.
Article26. Birken S, Maydelman Y, Gawinowicz MA, Pound A, Liu Y, Hartree AS. Isolation and characterization of human pituitary chorionic gonadotropin. Endocrinology. 1996; 137(4):1402–1411. PMID: 8625917.
Article27. Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, Domes G. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012; 37(9):1431–1438. PMID: 22365820.
Article28. Kimura T, Takemura M, Nomura S, Nobunaga T, Kubota Y, Inoue T, Hashimoto K, Kumazawa I, Ito Y, Ohashi K, Koyama M, Azuma C, Kitamura Y, Saji F. Expression of oxytocin receptor in human pregnant myometrium. Endocrinology. 1996; 137(2):780–785. PMID: 8593830.
Article29. Bossmar T, Osman N, Zilahi E, Haj MA, Nowotny N, Conlon JM. Expression of the oxytocin gene, but not the vasopressin gene, in the rat uterus during pregnancy: influence of oestradiol and progesterone. J Endocrinol. 2007; 193(1):121–126. PMID: 17400809.
Article30. Murata T, Narita K, Honda K, Higuchi T. Changes of receptor mRNAs for oxytocin and estrogen during the estrous cycle in rat uterus. J Vet Med Sci. 2003; 65(6):707–712. PMID: 12867731.
Article31. Murata T, Murata E, Liu CX, Narita K, Honda K, Higuchi T. Oxytocin receptor gene expression in rat uterus: regulation by ovarian steroids. J Endocrinol. 2000; 166(1):45–52. PMID: 10856882.
Article32. Novy MJ, Walsh SW. Dexamethasone and estradiol treatment in pregnant rhesus macaques: effects on gestational length, maternal plasma hormones, and fetal growth. Am J Obstet Gynecol. 1983; 145(8):920–931. PMID: 6837680.
Article33. Aman M, Hirano K, Nishimura J, Nakano H, Kanaide H. Enhancement of trypsin-induced contraction by in vivo treatment with 17beta-estradiol and progesterone in rat myometrium. Br J Pharmacol. 2005; 146(3):425–434. PMID: 16056237.34. Mesiano S, Welsh TN. Steroid hormone control of myometrial contractility and parturition. Semin Cell Dev Biol. 2007; 18(3):321–331. PMID: 17613262.
Article35. Parent M, Madore E, MacLaren LA, Fortier MA. 15-Hydroxyprostaglandin dehydrogenase in the bovine endometrium during the oestrous cycle and early pregnancy. Reproduction. 2006; 131(3):573–582. PMID: 16514200.
Article36. Matsuo M, Ensor CM, Tai HH. Characterization of the genomic structure and promoter of the mouse NAD+-dependent 15-hydroxyprostaglandin dehydrogenase gene. Biochem Biophys Res Commun. 1997; 235(3):582–586. PMID: 9207200.
Article37. Kelly RW, Linan C, Thong J, Yong EL, Baird DT. Prostaglandin inactivation is increased in endometrium after exposure to clomiphene. Prostaglandins Leukot Essent Fatty Acids. 1994; 50(5):235–238. PMID: 8066097.
Article38. Patel FA, Clifton VL, Chwalisz K, Challis JR. Steroid regulation of prostaglandin dehydrogenase activity and expression in human term placenta and chorio-decidua in relation to labor. J Clin Endocrinol Metab. 1999; 84(1):291–299. PMID: 9920098.
Article39. Dong YL, Yallampalli C. Pregnancy and exogenous steroid treatments modulate the expression of relaxant EP(2) and contractile FP receptors in the rat uterus. Biol Reprod. 2000; 62(3):533–539. PMID: 10684792.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bisphenol A and 4-tert-Octylphenol Inhibit Cx46 Hemichannel Currents
- The effects of etomidate on the contraction of pregnant rat uterine smooth muscle
- The Effects of Nitroglycerin on the Contraction of Rat Uterine Smooth Muscle
- Oxytocin and Oxytocin Antagonist Metabolism in the Plasma of Pregnant Women
- Problems Associated with I-125 Oxytocin Binding to Membrane Receptors