Lab Anim Res.
2014 Sep;30(3):104-111. 10.5625/lar.2014.30.3.104.
Evaluation of the genotoxicity of ginseng leaf extract UG0712
- Affiliations
-
- 1Laboratory of Genetic Toxicology, Korea Institute of Toxicology, KRICT, Daejeon, Korea.
- 2Department of Histology, College of Veterinary Medicine, Kyungpook National University, 1370 Sankyuk-dong, Bukgu, Daegu, Korea. psj26@knu.ac.kr
- 3Unigen Inc., Cheonan, Korea.
- KMID: 2312121
- DOI: http://doi.org/10.5625/lar.2014.30.3.104
Abstract
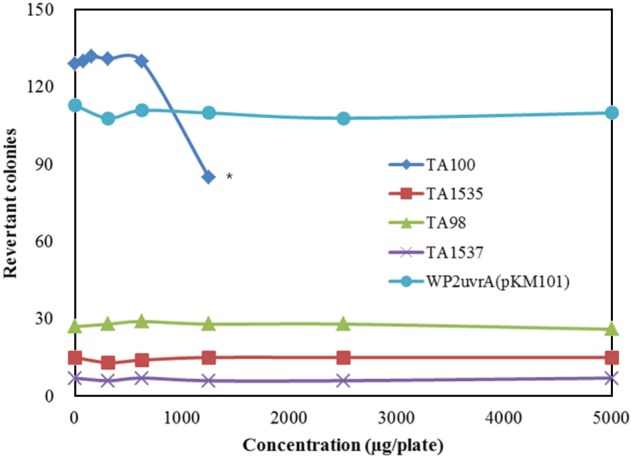
- Although ginseng (genus Panax) leaf extract contains high concentrations of bioactive constituents, its effects have been reported in few preclinical studies, and information regarding its toxicity is not sufficient to allow for its clinical use. We evaluated the genotoxicity of UG0712, which is a powdered extract of ginseng leaves. UG0712 did not increase the number of revertant colonies in 4 histidine auxotrophic strains of Salmonella typhimurium (TA100, TA1535, TA98, and TA1537) or in a tryptophan auxotrophic strain of Escherichia coli (WP2uvrA(pKM101)) at any concentration evaluated, either in the absence or presence of the metabolic activation system. There was no significant increase in the number of metaphase cells with structural or numerical aberrations in the UG0712-treated groups compared to the concurrent vehicle control at any dose, regardless of the presence of the metabolic activation system. Oral administration of the extract at doses up to 2,000 mg/kg in male mice did not increase the frequency of micronucleated polychromatic erythrocytes in the bone marrow, and did not result in any significant clinical signs, body weight loss, gross findings, or mortality. These results suggest that UG0712 does not act as a mutagenic or genotoxic material at the concentrations evaluated.
Keyword
MeSH Terms
Figure
Reference
-
1. Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999; 58(11):1685–1693. PMID: 10571242.2. Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005; 26(2):143–149. PMID: 15663889.
Article3. Shin HR, Kim JY, Yun TK, Morgan G, Vainio H. The cancer-preventive potential of Panax ginseng: a review of human and experimental evidence. Cancer Causes Control. 2000; 11(6):565–576. PMID: 10880039.4. Wang H, Peng D, Xie J. Ginseng leaf-stem: bioactive constituents and pharmacological functions. Chin Med. 2009; 4:20. PMID: 19849852.
Article5. Cheng TO. Panax (ginseng) is not a panacea. Arch Intern Med. 2000; 160(21):3329. PMID: 11088100.
Article6. Wang Z, Zheng Q, Liu K, Li G, Zheng R. Ginsenoside Rh(2) enhances antitumour activity and decreases genotoxic effect of cyclophosphamide. Basic Clin Pharmacol Toxicol. 2006; 98(4):411–415. PMID: 16623867.
Article7. Lee FC. Facts About Ginseng: The Elixir of Life. Hollym International Corporation;1992.8. Qi X, Ignatova S, Luo G, Liang Q, Jun FW, Wang Y, Sutherland I. Preparative isolation and purification of ginsenosides Rf, Re, Rd and Rb1 from the roots of Panax ginseng with a salt/containing solvent system and flow step-gradient by high performance counter-current chromatography coupled with an evaporative light scattering detector. J Chromatogr A. 2010; 1217(13):1995–2001. PMID: 20171644.
Article9. Raghavendran HR, Sathyanath R, Shin J, Kim HK, Han JM, Cho J, Son CG. Panax ginseng modulates cytokines in bone marrow toxicity and myelopoiesis: ginsenoside Rg1 partially supports myelopoiesis. PLoS One. 2012; 7(4):e33733. PMID: 22523542.
Article10. Ceylan-Isik AF, Fliethman RM, Wold LE, Ren J. Herbal and traditional Chinese medicine for the treatment of cardiovascular complications in diabetes mellitus. Curr Diabetes Rev. 2008; 4(4):320–328. PMID: 18991600.
Article11. Hou JP. The chemical constituents of ginseng plants. Comp Med East West. 1977; 5(2):123–145. PMID: 608333.
Article12. Zhang QH, Wu CF, Duan L, Yang JY. Protective effects of total saponins from stem and leaf of Panax ginseng against cyclophosphamide-induced genotoxicity and apoptosis in mouse bone marrow cells and peripheral lymphocyte cells. Food Chem Toxicol. 2008; 46(1):293–302. PMID: 17904265.
Article13. Xie JT, Mehendale SR, Wang A, Han AH, Wu JA, Osinski J, Yuan CS. American ginseng leaf: ginsenoside analysis and hypoglycemic activity. Pharmacol Res. 2004; 49(2):113–117. PMID: 14643691.
Article14. KFDA. Testing Guidelines for Safety Evaluation of Drugs (Notification No. 2014-136) issued by the Korea Food and Drug Administration. 2014.15. OECD. Guideline for Testing of Chemicals, Section 4, Health Effects, No. 471. 1997.16. Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983; 113(3-4):173–215. PMID: 6341825.
Article17. OECD. Guideline for Testing of Chemicals, Section 4, Health Effects, No. 473. 1997.18. Ishidate M Jr. Data book of chromosomal aberration test in vitro. Elsevier;1998.19. Ishidate M Jr, Odashima S. Chromosome tests with 134 compounds on Chinese hamster cells in vitro--a screening for chemical carcinogens. Mutat Res. 1977; 48(3-4):337–353. PMID: 876270.20. Sofuni T, Matsuoka A, Sawada M, Ishidate M Jr, Zeiger E, Shelby MD. A comparison of chromosome aberration induction by 25 compounds tested by two Chinese hamster cell (CHL and CHO) systems in culture. Mutat Res. 1990; 241(2):175–213. PMID: 2345556.
Article21. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65(1-2):55–63. PMID: 6606682.
Article22. OECD. Guideline for Testing of Chemicals, Section 4, Health Effects, No. 474. 1997.23. Hayashi M, MacGregor JT, Gatehouse DG, Adler ID, Blakey DH, Dertinger SD, Krishna G, Morita T, Russo A, Sutou S. In vivo rodent erythrocyte micronucleus assay. II. Some aspects of protocol design including repeated treatments, integration with toxicity testing, and automated scoring. Environ Mol Mutagen. 2000; 35(3):234–252. PMID: 10737958.
Article25. Kastenbaum MA, Bowman KO. Tables for determining the statistical significance of mutation frequencies. Mutat Res. 1970; 9(5):527–549. PMID: 5424720.
Article26. Ben-Hur E, Fulder S. Effect of Panax ginseng saponins and Eleutherococcus senticosus on survival of cultured mammalian cells after ionizing radiation. Am J Chin Med. 1981; 9(1):48–56. PMID: 7304498.
Article27. Keum YS, Han SS, Chun KS, Park KK, Park JH, Lee SK, Surh YJ. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutat Res. 2003; 523-524:75–85. PMID: 12628505.28. Liu WK, Xu SX, Che CT. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000; 67(11):1297–1306. PMID: 10972198.
Article29. Ong YC, Yong EL. Panax (ginseng)--panacea or placebo? Molecular and cellular basis of its pharmacological activity. Ann Acad Med Singapore. 2000; 29(1):42–46. PMID: 10748963.30. Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995; 18(9):1197–1202. PMID: 8845804.
Article31. Dey L, Xie JT, Wang A, Wu J, Maleckar SA, Yuan CS. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003; 10(6-7):600–605. PMID: 13678250.
Article32. Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002; 51(6):1851–1858. PMID: 12031973.
Article33. Xie JT, Zhou YP, Dey L, Attele AS, Wu JA, Gu M, Polonsky KS, Yuan CS. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine. 2002; 9(3):254–258. PMID: 12046868.
Article34. Wang BX, Cui JC, Liu AJ. The action of ginsenosides extracted from the stems and leaves of Panax ginseng in promoting animal growth. Yao Xue Xue Bao. 1982; 17(12):899–904. PMID: 7183121.35. EMEA/HMPC/107079, Guideline on the Assessment of Genotoxicity of Herbal Substances/Preparations. 2007.36. Abdelmigid HM. Sivakumar Gowder, editor. New Trends in genotoxicity testing of herbal medicinal plants, New Insights into Toxicity and Drug Testing. 2013. (ISBN: 978-953-51-0946-4, InTech, DOI: 10.5772/54858. Available from: http://www.intechopen.com/books/new-insights-into-toxicity-and-drug-testing/new-trends-in-genotoxicity-testing-of-herbal-medicinal-plants).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 13-week subchronic toxicity study of a novel ginsenoside composition from ginseng leaves in rats
- An Experimental Study on the Protective Effects of Ginseng Extract to Oxygen Toxicity
- Effect of Ginseng Extract on Blood Lipids and Atherosclerosis
- Mast Cell Degranulation with Special Reference to the Effect of a Saponin Extract of Ginseng upon the Mesenteric Mast Cell of Albino Rats
- Effect of Ginseng Extract on Male Rat Sexual Behavior