Korean J Urol.
2008 Apr;49(4):350-359.
The Effects of Human Muscle Derived Stem Cells on the Induction of Peripheral Nerve Regeneration
- Affiliations
-
- 1Department of Urology, College of Medicine, The Catholic University of Korea, Seoul, Korea. uroljy@catholic.ac.kr
Abstract
-
PURPOSE: In this study, we evaluated the extent of functional and histological axonal regeneration after resection of the sciatic nerve. The nerve was repaired with silicone tubes filled with human muscle derived stem cells(MDSCs) and neuronal progenitor cells(NPCs) in nude mice.
MATERIALS AND METHODS
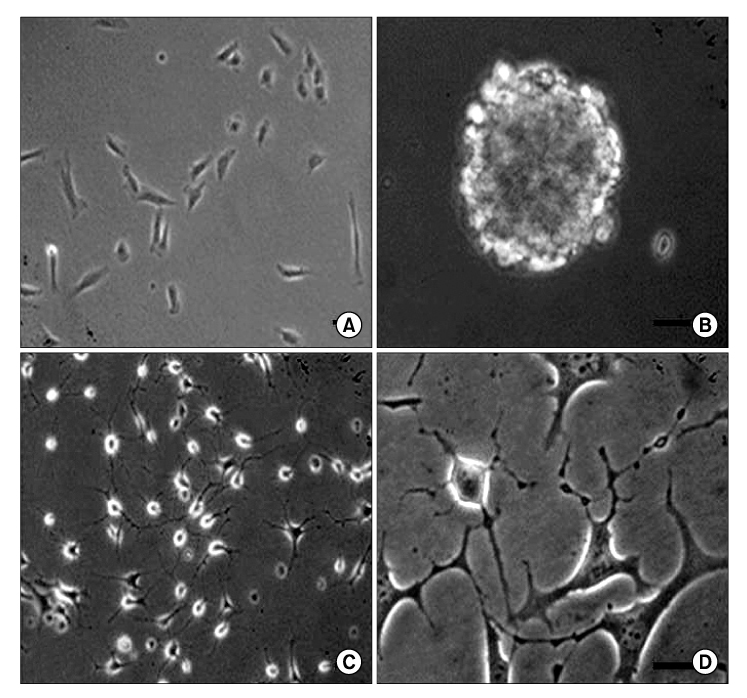
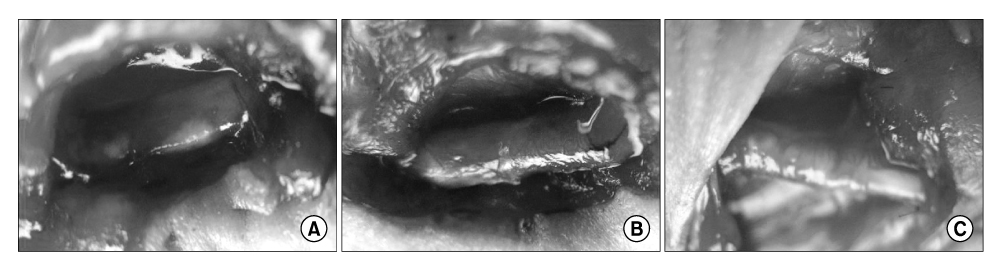
The human muscle samples were obtained from the rectus abdominis muscle of 12 patients that underwent a laparotomy. The MDSCs were isolated using a modified preplate technique. Using the MDSCs, neurogenic differentiation was induced by dissociating neurospheres produced in a neurosphere culture medium containing neuronal induction agents. A part of the sciatic nerve, approximately 7 mm in length, was excised bilaterally, and a 9mm long silicone tube guide was placed at the resulting gap in 40 nude mice. The transplanted sites were divided randomly into three groups according to the type of grafting cells: silicone tube guides filled with PBS(P group, n=20), silicone tube guides filled with MDSCs(M group, n=40) and silicone tube guides filled with NPCs(N group, n=20). Histological observations and a nerve conduction study were performed 12 weeks after the graft.
RESULTS
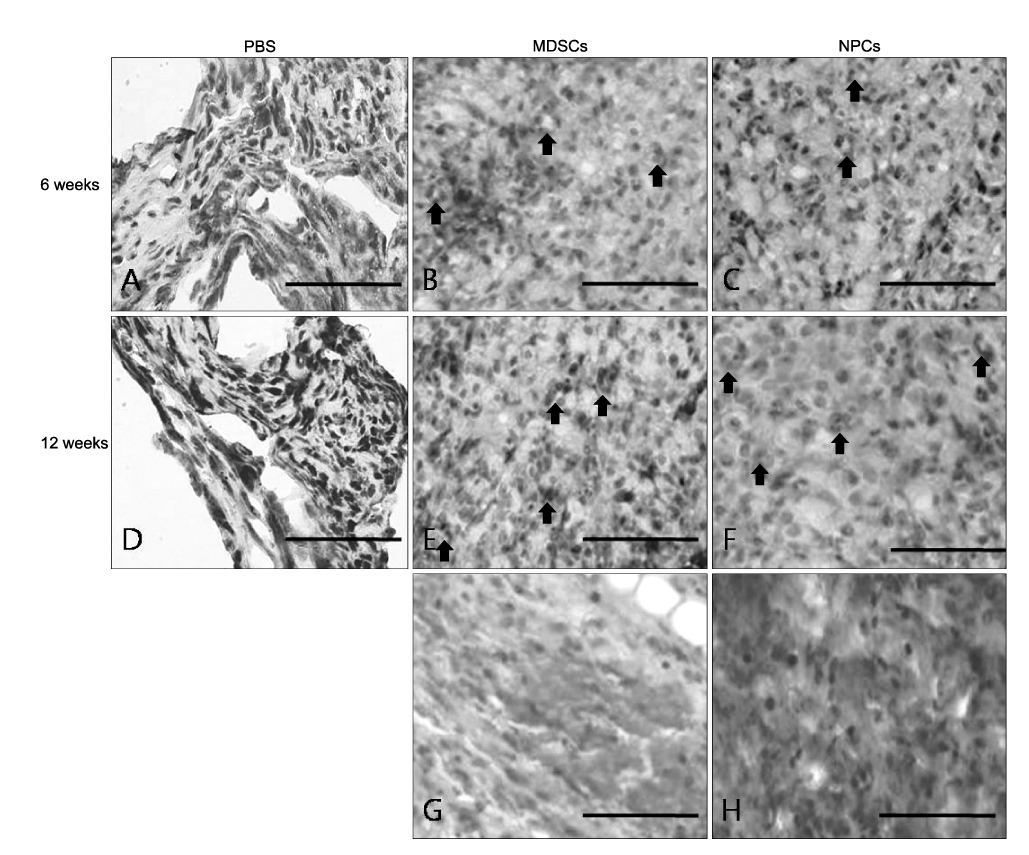
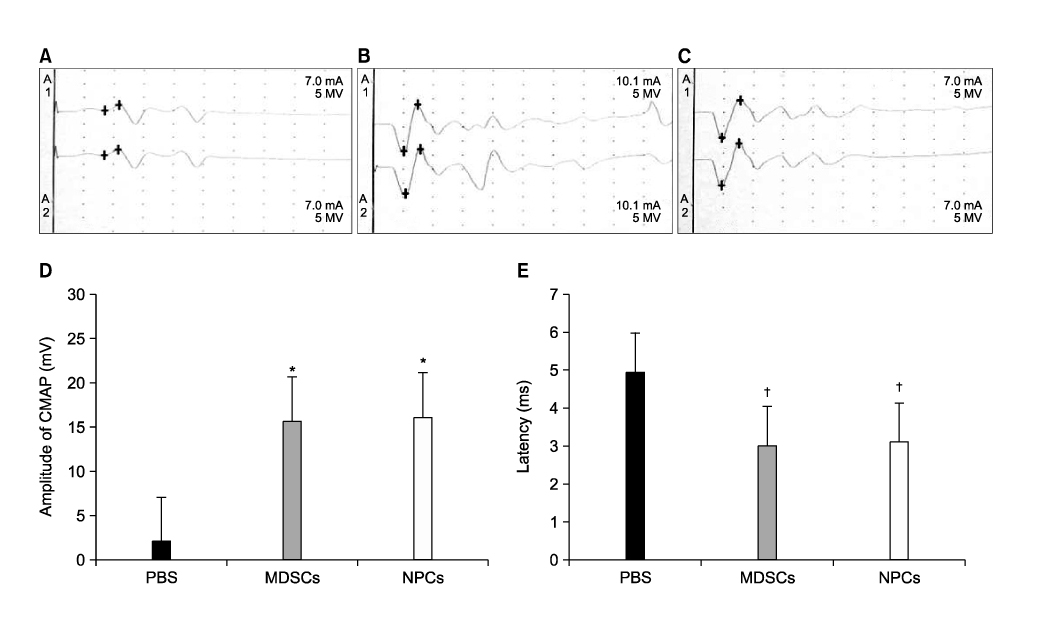
The number and diameter of the myelinated axons were significantly increased in the M and N groups(p<0.001). In a nerve conduction study, the amplitude of the compound muscle action potential(CMAP) and motor latency of response were significantly higher and shorter in the M and N groups(p<0.001). Moreover, reaction with neuronal class beta-tubulin(Tuj1, a neuronal marker) and antiglial fibrillary acidic protein(GFAP, a glial marker) was observed in the regenerated nerve that originated from the M and N groups.onclusions: These results show that MDSCs can differentiate into NPCs and improve the peripheral nerve regeneration rate after transplantation into a nerve guide.
Keyword
MeSH Terms
Figure
Reference
-
1. Nakao Y. An experimental study on the effect of laminin in vivo on promoting regeneration of axons. Nippon Seikeigeka Gakkai Zasshi. 1992. 66:334–349.2. Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990. 85:419–424.3. Bryan DJ, Wang KK, Chakalis-Haley DP. Effect of Schwann cells in the enhancement of peripheral-nerve regeneration. J Reconstr Microsurg. 1996. 12:439–436.4. Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998. 18:397–405.5. Peault B, Tavian M. Hematopoietic stem cell emergence in the human embryo and fetus. Ann N Y Acad Sci. 2003. 996:132–140.6. Daley GQ. From embryos to embryoid bodies: generating blood from embryonic stem cells. Ann N Y Acad Sci. 2003. 996:122–131.7. Fernandes KJ, McKenzie IA, Mill P, Smith KM, Akhavan M, Barnabe-Heider F, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004. 6:1082–1093.8. Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001. 3:778–784.9. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.10. Lin Y, Luo E, Chen X, Liu L, Qiao J, Yan Z, et al. Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. J Cell Mol Med. 2005. 9:929–939.11. Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002. 30:896–904.12. Muguruma Y, Reyes M, Nakamura Y, Sato T, Matsuzawa H, Miyatake H, et al. In vivo and in vitro differentiation of myocytes from human bone marrow-derived multipotent progenitor cells. Exp Hematol. 2003. 31:1323–1330.13. Romero-Ramos M, Vourc'h P, Young HE, Lucas PA, Wu Y, Chivatakarn O, et al. Neuronal differentiation of stem cells isolated from adult muscle. J Neurosci Res. 2002. 69:894–907.14. Alessandri G, Pagano S, Bez A, Benetti A, Pozzi S, Iannolo G, et al. Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet. 2004. 364:1872–1883.15. Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998. 142:1257–1267.16. McKinney-Freeman SL, Majka SM, Jackson KA, Norwood K, Hirschi KK, Goodell MA. Altered phenotype and reduced function of muscle-derived hematopoietic stem cells. Exp Hematol. 2003. 31:806–814.17. McKinney-Freeman SL, Jackson KA, Camargo FD, Ferrari G, Mavilio F, Goodell MA. Muscle-derived hematopoietic stem cells are hematopoietic in origin. Proc Natl Acad Sci U S A. 2002. 99:1341–1346.18. Wada MR, Inagawa-Ogashiwa M, Shimizu S, Yasumoto S, Hashimoto N. Generation of different fates from multipotent muscle stem cells. Development. 2002. 129:2987–2995.19. Peng H, Huard J. Muscle-derived stem cells for musculoskeletal tissue regeneration and repair. Transpl Immunol. 2004. 12:311–319.20. Hwang JH, Yuk SH, Lee JH, Lyoo WS, Ghil SH, Lee SS, et al. Isolation of muscle derived stem cells from rat and its smooth muscle differentiation. Mol Cells. 2004. 17:57–61.21. Sun JS, Wu SY, Lin FH. The role of muscle-derived stem cells in bone tissue engineering. Biomaterials. 2005. 26:3953–3960.22. Kondo T, Case J, Srour EF, Hashino E. Skeletal muscle-derived progenitor cells exhibit neural competence. Neuroreport. 2006. 17:1–4.23. Yannas IV, Hill BJ. Selection of biomaterials for peripheral nerve regeneration using data from the nerve chamber model. Biomaterials. 2004. 25:1593–1600.24. Guenard V, Aebischer P, Bunge RP. The astrocyte inhibition of peripheral nerve regeneration is reversed by Schwann cells. Exp Neurol. 1994. 126:44–60.25. Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000. 6:119–127.26. Paino CL, Fernandez-Valle C, Bates ML, Bunge MB. Regrowth of axons in lesioned adult rat spinal cord: promotion by implants of cultured Schwann cells. J Neurocytol. 1994. 23:433–452.27. Bunge MB, Bunge RP, Kleitman N, Dean AC. Role of peripheral nerve extracellular matrix in Schwann cell function and in neurite regeneration. Dev Neurosci. 1989. 11:348–360.28. Marchesi C, Pluderi M, Colleoni F, Belicchi M, Meregalli M, Farini A, et al. Skin-derived stem cells transplanted into resorbable guides provide functional nerve regeneration after sciatic nerve resection. Glia. 2007. 55:425–438.29. Ornelas L, Padilla L, Di Silvio M, Schalch P, Esperante S, Infante RL, et al. Fibrin glue: an alternative technique for nerve coaptation--Part II. Nerve regeneration and histomorphometric assessment. J Reconstr Microsurg. 2006. 22:123–128.30. Ornelas L, Padilla L, Di Silvio M, Schalch P, Esperante S, Infante PL, et al. Fibrin glue: an alternative technique for nerve coaptation--Part I. Wave amplitude, conduction velocity, and plantar-length factors. J Reconstr Microsurg. 2006. 22:119–122.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Adipose Derived Stem Cells on Neurogenic Differentiation and Induction of Nerve Regeneration
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Regenerative Strategies in Treatment of Peripheral Nerve Injuries in Different Animal Models
- In Vivo Effects of Adipose-Derived Stem Cells in Inducing Neuronal Regeneration in Sprague-Dawley Rats Undergoing Nerve Defect Bridged with Polycaprolactone Nanotubes
- Aucubin Promotes Neurite Outgrowth in Neural Stem Cells and Axonal Regeneration in Sciatic Nerves