Korean J Urol.
2008 Apr;49(4):313-319.
Initial Experiences of Intravesical Gemcitabine Instillation Followed by Bacillus Calmette-Guerin(BCG) Therapy for Treating Intermediate or High Risk Patients with Superficial Bladder Cancer
- Affiliations
-
- 1Department of Urology, College of Medicine, Korea University, Seoul, Korea. dkyoon@korea.ac.kr
Abstract
-
PURPOSE: To investigate the safety and the efficacy of intravesical gemcitabine therapy, we prospectively studied intravesical gemcitabine instillation followed by Bacillus Calmette-Guerin(BCG) instillation for treating the patients who suffer from superficial bladder cancer, and the above method was then compared with conventional BCG instillation.
MATERIALS AND METHODS
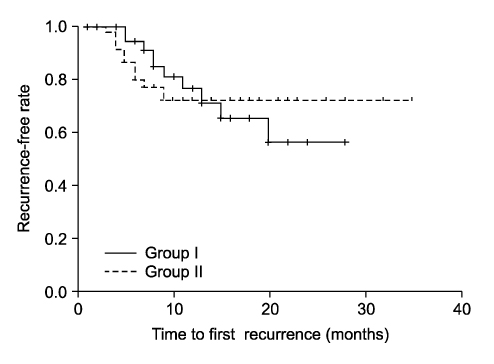
Between May 2005 and April 2007, a total of 84 patients were divided into Group I: the patients were treated with a 2-week course of gemcitabine(1,000mg/50ml, 2,000mg/50ml) followed by a conventional 6-week course of BCG, and Group II: the patients were treated by BCG instillation only. Gemcitabine was instilled immediately within 6 hours after complete trans-urethral resection of the bladder tumor (TURBT) and then this was repeated one week later. BCG instillation was started 2 weeks after TURBT. The complications, recurrence rates, progression rates and recurrence-free period(RFP) were analyzed in both groups.
RESULTS
The treatment was well tolerated in all the patients. Most of the complications were self-limiting, and there was no significant difference between the two groups(p=0.379). The recurrence rates of the two groups were 25.6% and 26.7%, respectively(p=0.899). Yet the recurrence-free period(RFP) was significantly longer in Group I(p=0.021). The progression rates of the two groups were 2.6% and 6.7%, respectively(p=0.620).
CONCLUSIONS
Intravesical gemcitabine instillation showed the effect to prolong the recurrence-free period for patients with superficial bladder cancer. Further long-term study will be needed.
MeSH Terms
Figure
Reference
-
1. Jones JS, Campbell SC. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Non-muscle-invasive bladder cancer (Ta, T1, and CIS). Campbell-Walsh urology. 2007. 9th ed. Philadelphia: Saunders;2447–2467.2. Hendricksen K, Witjes JA. Intravesical gemcitabine: an update of clinical results. Curr Opin Urol. 2006. 16:361–366.3. Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, Herr HW, et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol. 1992. 147:596–600.4. Gontero P, Marini L, Frea B. Intravesical gemcitabine for superficial bladder cancer: rationale for a new treatment option. BJU Int. 2005. 96:970–976.5. Oosterlinck W, Lobel B, Jakse G, Malmstrom PU, Stockle M, Sternberg C, et al. Guidelines on bladder cancer. Eur Urol. 2002. 41:105–112.6. Cho YH, Lee SJ, Kim CS, Lee ES, Hong SJ, Choi HY, et al. Safety and efficacy of intravesical Keyhole-Limpet Hemocyanin therapy for superficial transitional cell carcinoma: a prospective, multicenter study. Korean J Urol. 2006. 47:824–828.7. Hong SJ, Choi HY, Ahn HJ, Kim CS, Yang WJ. Effect of intravesical high dose epirubicin versus Bacillus Calmette-Guerin instillation on the recurrence and progression of superficial bladder cancer: a prospective, multicenter study. Korean J Urol. 2005. 46:677–682.8. Gontero P, Casetta G, Maso G, Sogni F, Pretti G, Zitella A, et al. Phase II study to investigate the ablative efficacy of intravesical administration of gemcitabine in intermediate-risk superficial bladder cancer (SBC). Eur Urol. 2004. 46:339–343.9. Serretta V, Galuffo A, Pavone C, Allegro R, Pavone-MacAluso M. Gemcitabine in intravesical treatment of Ta-T1 transitional cell carcinoma of bladder: Phase I-II study on marker lesions. Urology. 2005. 65:65–69.10. Gontero P, Frea B. Actual experience and future development of gemcitabine in superficial bladder cancer. Ann Oncol. 2006. 17:Suppl 5. 123–128.11. Gazzaniga P, Silvestri I, Gradilone A, Scarpa S, Morrone S, Gandini O, et al. Gemcitabine-induced apoptosis in 5637 cell line: an in-vitro model for high-risk superficial bladder cancer. Anticancer Drugs. 2007. 18:179–185.12. Witjes JA, van der Heijden AG, Vriesema JL, Peters GJ, Laan A, Schalken JA. Intravesical gemcitabine: a phase 1 and pharmacokinetic study. Eur Urol. 2004. 45:182–186.13. De Berardinis E, Antonini G, Peters GJ, Loves WJ, Van der Born K, Codacci-Pisanelli G, et al. Intravesical administration of gemcitabine in superficial bladder cancer: a phase I study with pharmacodynamic evaluation. BJU Int. 2004. 93:491–494.14. Gardmark T, Carringer M, Beckman E, Malmstrom PU. Randomized phase II marker lesion study evaluating effect of scheduling on response to intravesical gemcitabine in recurrent Stage Ta urothelial cell carcinoma of the bladder. Urology. 2005. 66:527–530.15. Dalbagni G, Russo P, Sheinfeld J, Mazumdar M, Tong W, Rabbani F, et al. Phase I trial of intravesical gemcitabine in bacillus Calmette-Guerin-refractory transitional-cell carcinoma of the bladder. J Clin Oncol. 2002. 20:3193–3198.16. Bassi P, De Marco V, Tavolini IM, Longo F, Pinto F, Zucchetti M, et al. Pharmacokinetic study of intravesical gemcitabine in carcinoma in situ of the bladder refractory to bacillus Calmette-Guerin therapy. Urol Int. 2005. 75:309–313.17. Oosterlinck W, Kurth KH, Schroder F, Bultinck J, Hammond B, Sylvester R. A prospective European Organization for Research and Treatment of Cancer Genitourinary Group randomized trial comparing transurethral resection followed by a single intravesical instillation of epirubicin or water in single stage Ta, T1 papillary carcinoma of the bladder. J Urol. 1993. 149:749–752.18. Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004. 171:2186–2190.19. Metwalli AR, Kamat AM. Controversial issues and optimal management of stage T1G3 bladder cancer. Expert Rev Anticancer Ther. 2006. 6:1283–1294.20. Manoharan M, Soloway MS. Optimal management of the T1G3 bladder cancer. Urol Clin North Am. 2005. 32:133–145.21. Palou J, Carcas A, Segarra J, Duque B, Salvador J, Garcia-Ribas I, et al. Phase I pharmacokinetic study of a single intravesical instillation of gemcitabine administered immediately after transurethral resection plus multiple random biopsies in patients with superficial bladder cancer. J Urol. 2004. 172:485–488.22. Soloway MS, Sofer M, Vaidya A. Contemporary management of stage T1 transitional cell carcinoma of the bladder. J Urol. 2002. 167:1573–1583.23. Kaasinen E, Rintala E, Pere AK, Kallio J, Puolakka VM, Liukkonen T, et al. Weekly mitomycin C followed by monthly bacillus calmette-guerin or alternating monthly interferon-alpha2B and bacillus Calmette-Guerin for prophylaxis of recurrent papillary superficial bladder carcinoma. J Urol. 2000. 164:47–52.24. Kaasinen E, Wijkstrom H, Malmstrom PU, Hellsten S, Duchek M, Mestad O, et al. Alternating mitomycin C and BCG instillations versus BCG alone in treatment of carcinoma in situ of the urinary bladder: a nordic study. Eur Urol. 2003. 43:637–645.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Intravesical High Dose Epirubicin versus Bacillus Calmette-Guerin Instillation on the Recurrence and Progression of Superficial Bladder Cancer: A Prospective, Multicenter Study
- Detection or Interleukin 2 In The Urine of Patients with Superficial Bladder Tumors after Intravesical BCG Therapy
- Evaluation of T-subsets and NK cell activity in patients with superficial bladder cancer after intravesical treatment with bacillus calmette-guerin
- The Effect of Lubricant on the Viabillty of Bacillus Calmette-Guerin
- Tuberculous Prostatic Abscess Following Intravesical Bacillus Calmette-Guerin Instillation