Korean J Urol.
2009 May;50(5):480-485.
The Effect of Human Muscle-Derived Stem Cells (MDSC) and Glycine-Isoleucine-Lysine-Valine-Alanine-Valine (GIKVAV) on the Cryo-Injured Bladder of Nude Mouse
- Affiliations
-
- 1Department of Urology, The Catholic University of Korea College of Medicine, Seoul, Korea. uroljy@catholic.ac.kr
- 2Department of Urology, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Biomaterials Research Center, Korea Institute of Science and Technology, Seoul, Korea.
Abstract
-
PURPOSE: In neurogenic bladder, both smooth muscle contraction and nerve regeneration are very important for functional improvement. Glycine-isoleucine-lysine-valine-alanine-valine (GIKVAV) is a peptide that can induce nerve regeneration in vivo. In this study, we evaluated bladder function after injection of muscle-derived stem cells (MDSCs) and GIKVAV into the cryo-injured bladder of nude mice.
MATERIALS AND METHODS
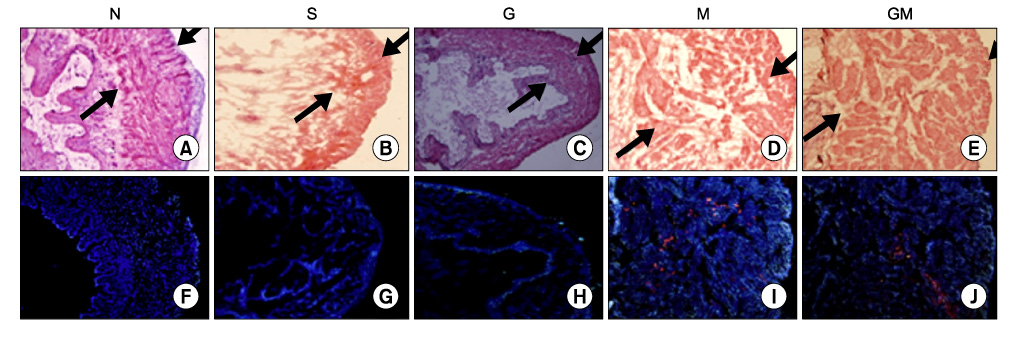
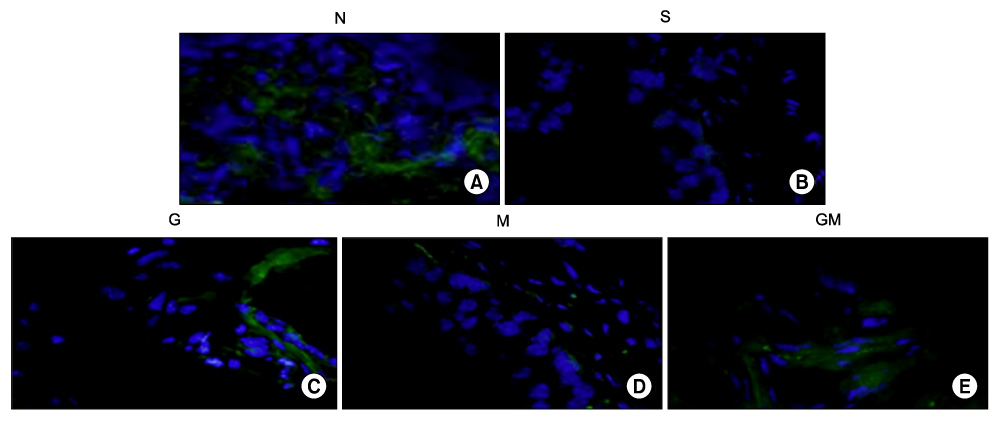
Human muscle samples were obtained from the rectus abdominis muscle of 12 patients who underwent laparotomy. The purpose and entire method of the study were explained to the patients, and all subjects who participated in this study provided written informed consent. The MDSCs were isolated by a modified preplate technique, and only CD34+ human MDSC were extracted by use of Mini-MACS kits. The nude mice were subdivided into 5 groups (n=40): normal group (N, n=8), saline injection group after cryo-injury (S, n=8), GIKVAV injection group after cryo-injury (G, n=8), human MDSC injection group after cryo-injury (M, n=8), and GIKVAV and human MDSC injection group after cryo-injury (GM, n=8). At 2 weeks after injection, we compared the contractility of a bladder muscle strip of each group by organ bath and polygraph by using electronic field stimulation (EFS). Nerve regeneration was evaluated by choline acetyl transferase (ChAT) immunostaining.
RESULTS
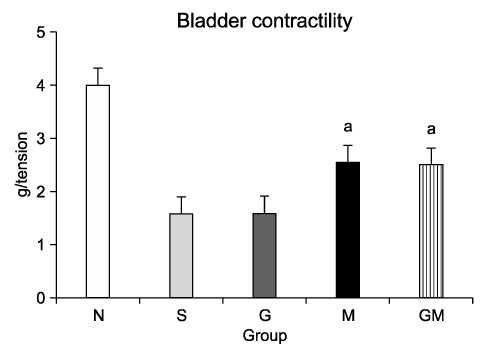
The contractile powers of the N, S, G, M, and GM groups were 3.58+/-0.27, 1.54+/-0.25, 1.54+/-0.31, 2.49+/-0.36, and 2.44+/-0.34 mN/mg, respectively, by EFS. The contractility of the bladder muscle strip in the S and G groups was lower than that in the N group. The contractile powers of the M and GM groups were lower than those of the N group but greater than those of the S and G groups. In ChAT immunohistochemical staining, nerve regeneration was increased in the G and GM groups compared with the S and M groups.
CONCLUSIONS
Nerve regeneration was induced by GIKVAV injection regardless of human MDSC injection. There was no direct effect of GIKVAV on bladder muscle contractility.
Keyword
MeSH Terms
Figure
Reference
-
1. Koh JS, Lee JY, Lee JY. The effects of human muscle derived stem cells on the Induction of peripheral nerve regeneration. Korean J Urol. 2008. 49:350–359.2. Lee HN, Lee JY, Koh JS, Kim HW, Byun SS, Lee SS, et al. Muscle derived stem celll/alginate/polycaprolactone/ injection therapy in rats with denervated urethral sphincter. Korean J Urol. 2007. 48:1296–1301.3. Choo GY, Lee JY, Park WH, Jung YS. Effects of injection therapy using muscle derived stem cell/chitosan/hydroapatite composite gel in a rat model of urinary incontinence. Korean J Urol. 2007. 48:627–632.4. Byun SS, Chung YS, Lee SS, Lee HN, Lee JY, Lee JY. Augmentation cystoplasty using hydroxapatite/chitosan composite sheet seeded with autologous muscle-derived stem cells. Korean J Urol. 2007. 48:433–438.5. Lin X, Takahashi K, Liu Y, Zamora PO. Enhancement of cell attachment and tissue integration by a IKVAV containing multi-domain peptide. Biochim Biophys Acta. 2006. 1760:1403–1410.6. Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998. 142:1257–1267.7. Reali C, Scintu F, Pillai R, Cabras S, Argiolu F, Ristaldi MS, et al. Differentiation of human adult CD34+ stem cells into cells with a neural phenotype: role of astrocytes. Exp Neurol. 2006. 197:399–340.8. Somogyi GT, Yokoyama T, Szell EA, Smith CP, de Groat WC, Huard J, et al. Effect of cryoinjury on the contractile parameters of bladder strips: a model of impaired detrusor contractility. Brain Res Bull. 2002. 59:23–28.9. Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003. 62:958–963.10. Lee JY, Cannon TW, Pruchnic R, Fraser MO, Huard J, Chancellor MB. The effects of periurethral muscle-derived stem cell injection on leak point pressure in a rat model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2003. 14:31–37.11. Huard J, Yokoyama T, Pruchnic R, Qu Z, Li Y, Lee JY, et al. Muscle-derived cell-mediated ex vivo gene therapy for urological dysfunction. Gene Ther. 2002. 9:1617–1626.12. Gunn JW, Turner SD, Mann BK. Adhesive and mechanical properties of hydrogels influence neurite extension. J Biomed Mater Res A. 2005. 72:91–97.13. Tysseling-Mattiace MV, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008. 28:3814–3823.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Amino Acid Compositions of Formula for Children with Inherited Metabolic Disorder
- Serum Amino Acid Levels in Term and Preterm Neonates
- Nutritional status and plasma amino acid profile in maintenance hemodialysis patients
- Age Specific Reference Values of 24 Plasma Free Amino Acids in Korean Children
- Paper chromatographic study on the amino acids of some parasitic helminths